Treatment of various blood cancers, PI3K oral inhibitors submitted for marketing application
Treatment of various blood cancers, PI3K oral inhibitors submitted for marketing application
February 08, 2018 Source: WuXi PharmaTech
Window._bd_share_config={ "common":{ "bdSnsKey":{ },"bdText":"","bdMini":"2","bdMiniList":false,"bdPic":"","bdStyle":" 0","bdSize":"16"},"share":{ }};with(document)0[(getElementsByTagName('head')[0]||body).appendChild(createElement('script')) .src='http://bdimg.share.baidu.com/static/api/js/share.js?v=89860593.js?cdnversion='+~(-new Date()/36e5)];Today, Verastem announced that it has submitted a listing application for the leading drug candidate duvelisib to the US FDA. Verastem expects this new drug to be fully approved for the treatment of relapsed or refractory chronic lymphocytic leukemia/small lymphocytic lymphoma, as well as for accelerated approval of relapsed or refractory follicular lymphoma.

Duvelisib is a first-in-class new drug that inhibits both PI3K-delta and PI3K-gamma. These two enzymes play a key role in the growth and survival of malignant B cells and T cells: their signaling pathways cause the proliferation of malignant B cells and T cells, and may also play a role in the formation and maintenance of the tumor microenvironment. As a new drug with innovative mechanisms, researchers are evaluating the efficacy of duvelisib in a number of clinical trials. Based on the positive results demonstrated in clinical trials, duvelisib has been awarded the fast track qualification and orphan drug status by the US FDA.
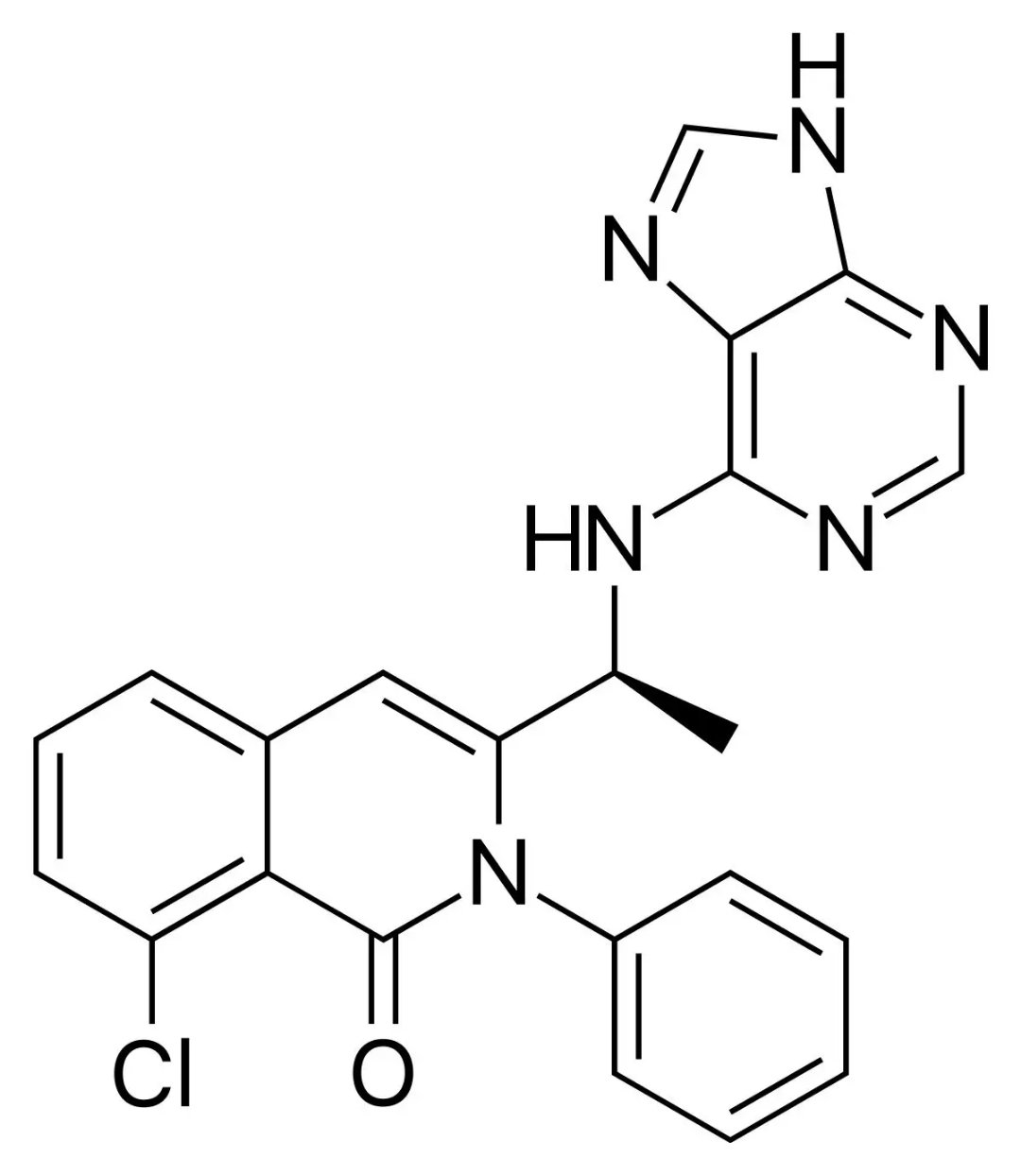
â–²Duvelisib's molecular structure (Source: Wikipedia)
The efficacy and safety of duvelisib was further validated in a phase 3 clinical trial called DUO. The trial enrolled patients with relapsed or refractory chronic lymphocytic leukemia/small lymphocytic lymphoma and received monotherapy with duvelisib. Studies have shown a statistically significant prolongation of progression-free survival (PFS) in patients receiving duvelisib compared with controls using theatumumab (median PFS in the treatment group: 13.3 months, median PFS in the control group: 9.9 months; HR = 0.52; p < 0.0001), the risk of progression or death of the patient was reduced by 48%.
In another phase 2 clinical trial called DYNAMO, the researchers also analyzed the efficacy of duvelisib in the treatment of inert non-Hodgkin's lymphoma. In this study, patients receiving treatment developed dual resistance to rituximab and chemotherapy/radioimmunotherapy. Among these patients who lacked treatment options, duvelisib achieved an objective response rate (ORR) of 46% (p < 0.0001), reaching the primary clinical endpoint. In the subgroup of patients with dual-resistance follicular lymphoma, the ORR of duvelisib also reached 41%.
Based on the excellent results of these two clinical trials, Verastem submitted a duvelisib listing application to the US FDA.
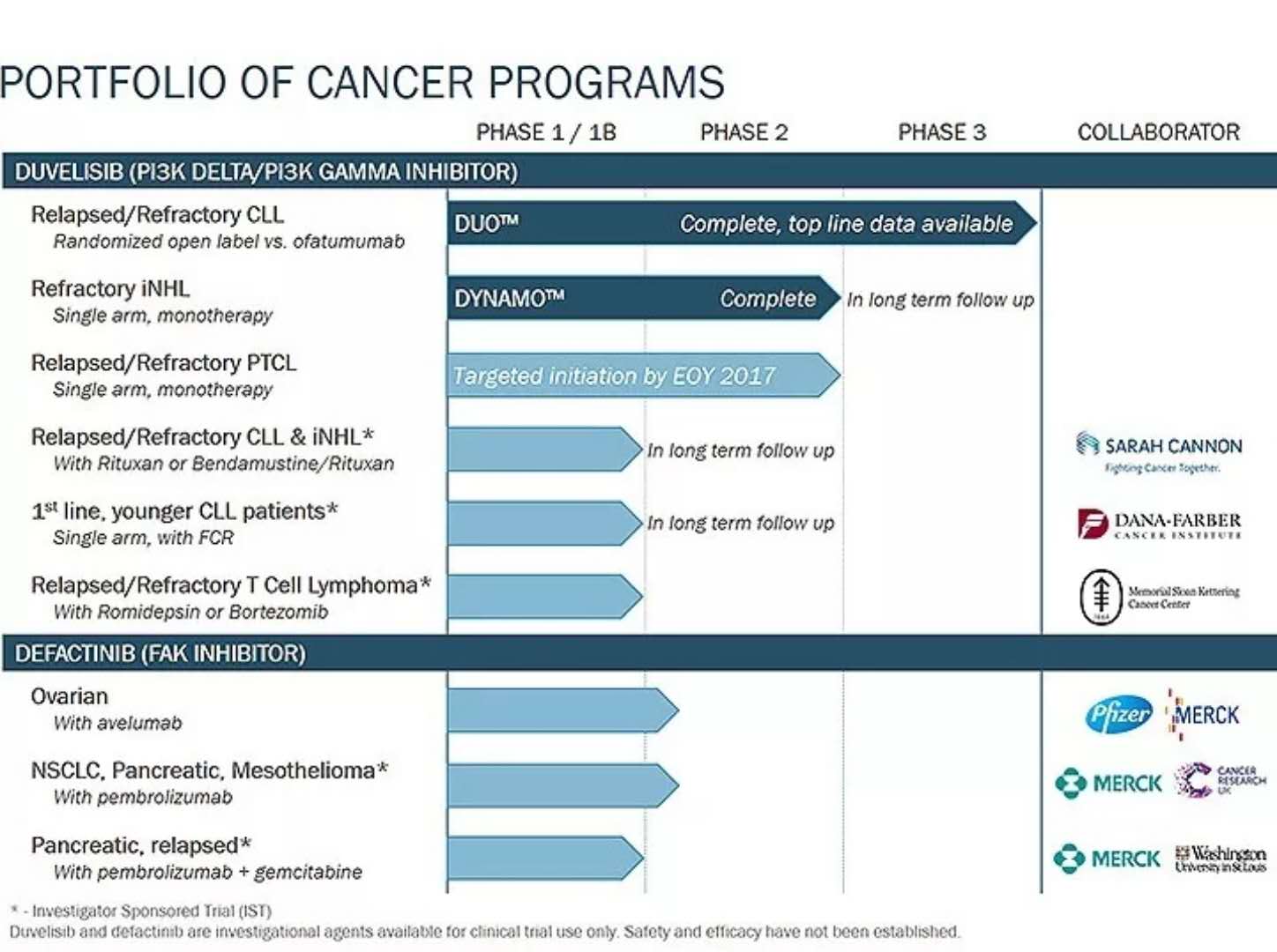
â–²Verastem's R&D pipeline (Source: Verastem official website)
“The submission of the Duvelisib listing application is an important milestone for Verastem and the result of continued efforts by our staff and researchers. They are dedicated to developing new potential treatment options for patients who require additional therapies. We have also been involved in duvelisib clinical practice for the past few years. The patients in the trial project expressed their sincere gratitude," said Verform's President and CEO, Robert Forrester: "Duvelisib is the first monotherapy in patients with relapsed or refractory chronic lymphocytic leukemia/small lymphocytic lymphoma. Oral PI3K inhibitors that show efficacy. As monotherapy, it also shows significant clinical results in patients with dual-resistance follicular lymphoma. We believe that duvelisib can provide a convenient oral treatment regimen and is also expected to be with the FDA. Working together during the review process, the new drug is expected to be approved in early 2019."
We look forward to the availability of this new drug as soon as possible for the benefit of these patients who lack treatment.
Reference materials:
[1] Verastem Submits New Drug Application to US FDA for Duvelisib for the Treatment of Patients with Relapsed or Refractory Chronic Lymphocytic Leukemia/Small Lymphocytic Lymphoma and Follicular Lymphoma
Guangzhou Zhongzhinan Supply Chain Co.,Ltd. , https://www.zhongzhinanlighting.com