The dry eye syndrome that has been scorned domestically is popular in foreign countries. What are the characteristics of these five companies that treat dry eye syndrome?
On June of the National Eyes Day in June 2018, the National Health Council held a press conference to introduce prevention and control of myopia. At the same time, the "Guidelines for Prevention and Control of Myopia", "Guidelines for Diagnosis and Treatment of Amblyopia" and "Guidelines for Diagnosis and Treatment of Strabismus" were also released. At the end of the conference, Long Qin, a professor of ophthalmology at Peking Union Medical College Hospital, emphasized that in the prevention and control of myopia, the problem that has not been paid attention to is the problem of dry eye syndrome among adolescents. At present, there are still problems of improper treatment.
However, in the clinical dry eye syndrome has become the second largest eye disease in addition to refractive, according to relevant data, the number of dry eye patients in China has surged, growing at a rate of 10% per year. The incidence of dry eye in developed countries is about 15% to 16%, while the incidence of dry eye in China is 21%.
In the absence of sufficient attention in the country, the arterial network found that the treatment of dry eye related in foreign markets was popular. In the short-term, the related drug equipment research and development will be able to obtain the olive branch from the pharmaceutical giant. A large amount of capital has also poured into it to promote research and development.
Dry eye syndrome market lacks new drugs, waiting for new drug research and development
Dry eye syndrome is divided into pathological dry eye syndrome and dry eye syndrome caused by poor eye habits. The initial symptoms of dry eye syndrome are dry eyes. If not treated promptly and effectively, it is easy to develop into intractable dry eye, form keratitis and corneal ulcer, and even cause blindness. In the United States, there are approximately 340 million patients with chronic dry eye disease. However, there are fewer drugs for treating dry eye. Artificial tears can only stay on the surface of the eye and are completely discharged within 15 minutes.
In 2016, when Shire launched the eye drug Xiidra (lifitegrast eye drops) for dry eye disease, it was the first drug in the US market to receive FDA-approved dry eye syndrome in the past 13 years. It was the first in the US market. a prescription eye drops. With the advancement of technology, the treatment of dry eye treatment is not limited to external eye drops treatment, and a large number of new drugs and equipment have emerged in foreign markets.
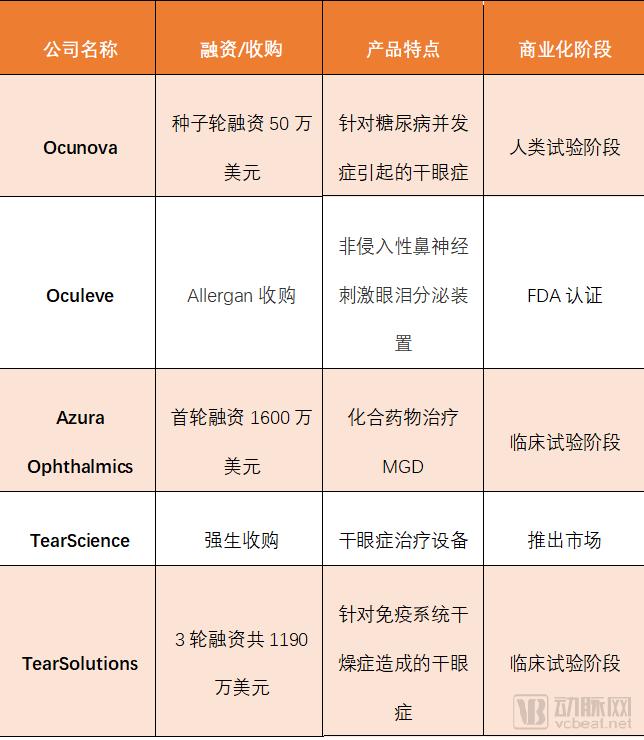
The arterial network found five start-up companies ranging from equipment to medications for all types of dry eye syndrome. It is found that not only do many new drug research and development markets enter the market for dry eye disease research and development, but many giants also join the market by acquiring technology-developed start-ups.
Ocunova: old drugs cure new diseases, for dry eye syndrome
On June 6, the biotech startup Ocunova seed round received $500,000 in financing to support the development of clinical trials for the treatment of dry eye syndrome caused by diabetes. The therapy includes an FDA-approved drug for managing alcohol and opioid dependence. It is innovatively used to treat dry eye caused by diabetes. Ocunova gained a high level of interest abroad because of the development of new uses in drugs approved by the FDA.
Chronic dry eye syndrome in diabetic patients is 20 million in the United States and 50 million worldwide. Current treatments are limited to over-the-counter eye drops or two prescription and anti-inflammatory drugs for temporary relief of the eye, but these drugs only serve as a delay and cause side effects, which limits patient compliance.
It is different from the lubricants commonly used in the treatment of dry eye products, and the tear-like moisturizing products and anti-inflammatory drugs. Ocunova provides medications for specific diabetic dry eye patients. Ocunova's new eye drops are patented.
At present, eye drops have passed safety tests in various animal tests, and the safety and effectiveness of OCU-01 in human clinical trials will be further evaluated. It is also the direction that this seed round financing will be applied.
The reason for this investment, in addition to product innovation, the investment sector is optimistic about the local innovation ecosystem embodied by Ocunova.
When it comes to investing in the company, Dr. Mel Billingsley, president and CEO of LSGPA, one of the investors, said: "This investment is the best example of the Pennsylvania innovation ecosystem. Ocunova was founded by local entrepreneurs. The core technology is A world-class technology developed by researchers at the Pennsylvania State University School of Medicine." LSGCP is a national technology transformation organization that also works with other agencies to explore and develop life-supporting technology companies.
Osunova CEO Michael Shine was the chief consultant of Novanta. Also a vice president of Wyeth Pharmaceuticals, Wyeth Pharmaceuticals has been acquired by Ruihui.
Two professors in the Ocunova core team
TearSolutions: CEO successfully incubating two ophthalmic projects
TearSolutions claims to offer natural remedies for dry eye syndrome, and the company's treatment for dry eye is mainly for dry eye syndrome caused by initial dryness syndrome. The purpose of treatment is to restore the health of the surface of the eye, and to treat symptoms such as dryness, burning, and discomfort caused by dry eye syndrome of the primary dryness syndrome. This includes repairing the nerves on the cornea and letting the brain produce more tears. It also stabilizes the tear film.
Primary xerodiarrhea is an immune system disorder that is already a common immune system disease in the United States. Currently, approximately 4 million people in the United States suffer from xerostomia. There is currently no cure, and treatment can improve symptoms and prevent complications.
The product Lacripep? provided by TearSolutions is a component of normal human tears, but dry eye patients lack this ingredient in tears. In clinical trials, Lacripep®, developed by TearSolutions, restores tear secretion and eye health. TearSolutions is currently in the process of recruiting patients for clinical trials to evaluate the safety and efficacy of Lacripep®.
TearSolutions founder team's resume is more eye-catching than the product. The experience of the founder team's many successful startups and the experience of the giant pharmaceutical company executives can also bring more protection to investment.
Tom R., Chairman of TearSolutions Gadek's last CEO, SARcode, was acquired by Charles in 2013 for 160 million. In 2016, after acquiring SARcode, Charles introduced Lifitegrast, the core product for dry eye, to Xiidra, which became the core product for the treatment of dry eye disease.
Executive Chairman Mark B. Logan is the former chairman of VISX, a pioneer in the global excimer laser eye vision correction technology. The entrepreneurial team also includes Professor of Cell Biology and Ophthalmology from the University of Virginia, Gordon W. Laurie. After three rounds of financing, the total financing was $11.9 million.
Azura Ophthalmics: Pushing dry eye treatment to the next stage
Azura Ophthalmics is a clinically-based biotechnology company headquartered in Javert, Tel Aviv, Israel. Azura Ophthalmics is developing an innovative combination of compounds to promote meibomian gland dysfunction (MGD), the main cause of dry eye syndrome.
After Gutgesell et al first proposed meibomian gland dysfunction (MGD) in 1982, this concept gradually gained widespread acceptance. Although clinically very common, it is often overlooked and the efficacy of existing therapies is not significant.
The founders said that they have hatched a number of projects acquired by giants, and the existing management has rich marketing experience. Founder Dr. Yair Alster co-founded ForSight Vision4. The company was founded by ForSight Labs, an ophthalmology incubator, and was acquired by Roche Genes in 2017. He then co-founded ForSight Vision5 and was eventually acquired by Allergan like the previous company. CEO Marc Gleeson served as Vice President of Global Ophthalmology Marketing at Allergan for 14 years before joining Oculeve.
The first round of financing received $16 million in financing. Major investors including OrbiMed, TPG Biotech, and Brandon Capital jointly invested in Ganot Capital.
TearScience: Another treatment for dry eye from equipment treatment
Like Azura Ophthalmics, TearScience is also a meibomian gland to improve dry eye. Different from the above three treatments for dry eye from the drug treatment route. TearScience improves meibomian gland function from medical devices .
According to the official website, the treatment equipment of tearscience includes LipiFlow, LipiScan, and LipiView II, which are three kinds of dry eye treatment and imaging equipment.
LipiFlow uses a drug-free mechanism that transfers heat and pressure to the eyelids to help clear blocked glands. VTP? uses unique heat and massage treatments to enhance gland function. Use LipiScan and LipiView II to provide accurate gland morphological visualization using non-contact surface illumination and high definition transillumination. TearScience fell into Johnson and Johnson last year.
Johnson & Johnson said that after choosing to acquire tearscience, it hopes to integrate with Johnson & Johnson's existing ophthalmic business to meet consumers' more eyesight needs.
From the company's official website, TearScience was found to provide eyelid imaging equipment and heat pulse dry eye treatment equipment. Eighty-six percent of patients with dry eye can improve diaphragmatic gland function and dry eye symptoms after using the LipiFlow program. The Meibomian Gland Evaluator? (MGE) provides a standardized, repeatable evaluation of meibomian gland function. LipiScan? & LipiView II? provides high-definition meibography? and dynamic seesaw imaging.
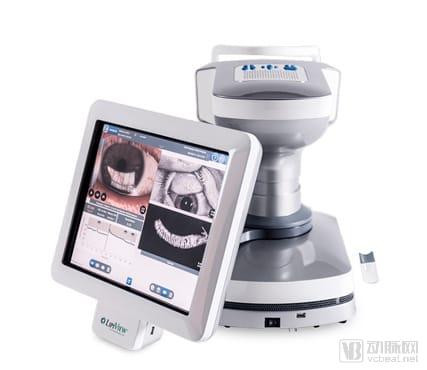
TearScience Eye Imaging Equipment
Oculeve: FDA-approved dry eye treatment device
The dry eye market is quite active, in addition to the innovations in drug therapy described above, there is also a small and flexible treatment device. This non-invasive nasal nerve stimulation device was named OD-01.
Oculeve hatched a biodesign project at Stanford University that developed a tool to increase tears by stimulating the nasal nerves and received investments from Kleiner Perkins Caulfield, Bayer, New Enterprise Associates (NEA) and Versant Ventures. Allergan acquired Oculeve for $125 million in 2015. Alfred hopes that this move will strengthen its leading position in the global eye market. In April last year, Oculeve products received an FDA-approved commercialization license.
David Nicholson, executive vice president of Allergan, said: "Allergan is committed to developing a range of innovations to help patients with dry eye syndrome."

Oculeve completed four clinical trials of OD-01 in more than 200 patients at the time of the acquisition, indicating that the device is positively safe and effective. Ai Jian joined before submitting the FDA application and conducted two key tests.
Core technology plus operation team, more easily favored by capital giants
It can be seen that in terms of technology, capital and giants prefer technology maturity and certified dry eye treatment technology. Although the dry eye treatment market is now monopolized by artificial tears and several drugs, the best way to cut into the market is to target Development of drugs for dry eye caused by specific causes. It has been reported in the country that patients directly purchase over-the-counter Japanese eye drops and cause corneal diseases. Adolescents also tend to treat dry eye syndrome as a conjunctivitis to aggravate symptoms.
Cooperate with scientific research institutions to commercialize cash technology and make it easier to obtain support. From the composition of several companies' entrepreneurial teams, it can be seen that most of the portfolios are based on the model of a large pharmaceutical company CEO plus research team. In addition to ensuring the ability to develop new drugs or equipment, superior marketing capabilities are also essential to the commercialization of technology, and experienced people are often able to achieve rapid technical implementation and realisation.
At the same time, dry eye syndrome has a lot of room to explore. FDA officials have said that dry eye disease is more common in children, and the safety and effectiveness of many new drugs have not been studied in patients under 17 years old. .
Medical Equipment Disposal,Syringes Needles Sizes,Disposable Syringe,Insulin Syringe
FOSHAN PHARMA CO., LTD. , https://www.foshanpharma.com