For the BRAF resistance, Merck's new drug shows early efficacy
For the BRAF resistance, Merck's new drug shows early efficacy
February 24, 2018 Source: WuXi PharmaTech
Window._bd_share_config={ "common":{ "bdSnsKey":{ },"bdText":"","bdMini":"2","bdMiniList":false,"bdPic":"","bdStyle":" 0","bdSize":"16"},"share":{ }};with(document)0[(getElementsByTagName('head')[0]||body).appendChild(createElement('script')) .src='http://bdimg.share.baidu.com/static/api/js/share.js?v=89860593.js?cdnversion='+~(-new Date()/36e5)];Researchers at the University of North Carolina (UNC) Lineberger Comprehensive Cancer Center and other partner agencies reported that a new drug, Merck (MSD), has shown efficacy in early clinical trials of advanced melanoma and other drug-resistant cancers.

Although targeted therapies have been approved for melanoma and lung cancer with specific mutations in the BRAF gene, most patients develop resistance to these treatments and cancers recur, with the most common cause being ERK reactivation. To overcome the resistance of cancer cells to anti-BRAF drugs, researchers at UNC Lineberger and other institutions have studied MK-8353 compounds to block ERK.
MK-8353 is an oral, bioavailable, and selective potent ERK inhibitor that inhibits activated ERK1 and ERK2 in vitro. MK-8353 can proportionally reduce the activation of ERK1 (pERK1), ERK2 (pERK2) and ribosomal S6 kinase (pRSK) protein phosphorylation. In A2058 cells, 30 nanomoles of MK-8353 completely inhibited pERK1 and pERK2. In addition, MK-8353 can also inhibit the proliferation of a group of BRAFV600 mutant and RAS mutant cancer cells in vitro.
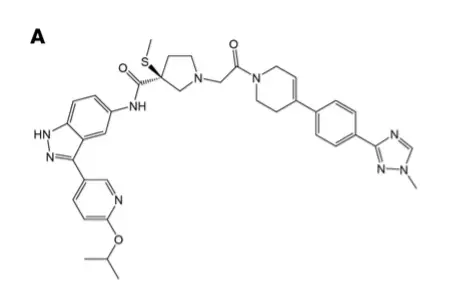
â–²MK-8353 molecular structure (Source: "JCI Insight")
The drug was clinically tested in Phase 1 in healthy volunteers (P07652) and advanced solid tumor patients (MK-8353-001). In the P07652 study conducted in the Netherlands, 48 ​​healthy volunteers were given a single oral dose of 10 to 400 mg of MK-8353. In the MK-8353-001 study, 26 cancer patients were orally administered 100 to 800 mg doses of MK-8353 twice daily. These studies analyzed the safety, tolerability, pharmacokinetics, pharmacodynamics and antitumor activity of the drug.
MK-8353 was generally safe and well tolerated in all doses and no serious adverse events (SAE) occurred. Adverse events in P07652 included diarrhea (44%), fatigue (40%), nausea (32%) and rash (28%). Dose-limiting toxicity was observed in the 400 mg and 800 mg dose groups. Of the 15 patients who were evaluated for treatment remission in the MK-8353-001 study, 3 had partial remission, all of whom had BRAFV600 mutant melanoma.
“ERK stimulates factors that promote cancer growth,†said Dr. Channing Der, a professor of pharmacology at the UNC School of Medicine. “ERK is very complex, and our understanding of it is still very small. It is clear that it is essential for cancer growth. This is why there are many inhibitors approved or undergoing clinical evaluation in this pathway. Obviously, pharmaceutical companies have good reason to believe that this is an important biological pathway, let us study inhibitors to fight it. â€
We look forward to seeing more positive data in the near future, supporting this new drug as a promising anti-cancer drug.
Reference materials:
[1] Merck's melanoma drug shows early promise for resistant cancers in PhI trial
[2] Phase I clinical trial shows some promise for investigational drug for melanoma
[3] Development of MK-8353, an orally administered ERK1/2 inhibitor, in patients with advanced solid tumors
Shaanxi Kang New Pharmaceutical co., Ltd. , https://www.apipepdites.com