Scientists Analyze the Complex Structure of Farnesol X Receptor and Retinoic Acid X Receptor, Important Targets of Metabolic Diseases
Scientists Analyze the Complex Structure of Farnesol X Receptor and Retinoic Acid X Receptor, Important Targets of Metabolic Diseases
October 17, 2018 Source: Chinese Academy of Sciences
Window._bd_share_config={ "common":{ "bdSnsKey":{ },"bdText":"","bdMini":"2","bdMiniList":false,"bdPic":"","bdStyle":" 0","bdSize":"16"},"share":{ }};with(document)0[(getElementsByTagName('head')[0]||body).appendChild(createElement('script')) .src='http://bdimg.share.baidu.com/static/api/js/share.js?v=89860593.js?cdnversion='+~(-new Date()/36e5)];Recently, Liu Jinsong, from the Guangzhou Institute of Biomedicine and Health, Chinese Academy of Sciences, analyzed the complex structure of FXR and FXR/RXR complexes with different types of agonists by structural biology and other methods to adjust the structure of FXR/RXR complexes. The phenomenon was studied, and the related results were published online in the Journal of Biological Chemistry on October 1st under the title of Ligand binding and heterodimerization with retinoid X receptor α (RXRα) induce farnesoid X receptor (FXR) conformational changes affecting co-activator binding.
In recent years, a disease group called metabolic syndrome, including hyperlipidemia and insulin resistance, has gradually become a major disease that endangers human health. Because its pathogenesis is unknown, there is still no effective means for the treatment of metabolic syndrome. Recent studies have found that certain nuclear receptor transcription factors can participate in the regulation of multiple metabolic pathways through the regulation of their corresponding target genes, and the research progress of farnesyl alcohol X receptor (FXR) is the treatment of various metabolic diseases. Provide new ideas and directions. FXR is a bile acid receptor and a member of the nuclear receptor superfamily. FXR has important regulatory effects on cholesterol metabolism, lipid homeostasis, and dietary fat and Vitamin absorption through regulation of target gene expression, and is therefore a metabolic disease (eg, primary biliary cirrhosis and nonalcoholic fat). An important drug target for sexual hepatitis. In most cases, FXR and retinol X receptor (RXR) bind to the FXR response element of the target gene promoter region in a heterodimeric form to regulate transcription of downstream genes, while FXR/RXR heterodimers are currently present. The structure and function are still unclear. Therefore, the study of complex structure studies of FXR and RXR can provide a structural basis and direction for the development of selective FXR/RXR agonists.
This study found the flexibility of the FXR ligand binding pocket by comparing the structure of different agonists to the FXR complex, enabling it to bind to a diverse array of small molecules. The study also showed that the FXR/RXR heterodimeric complex has a higher affinity for coactivators than when they are each monomer. Binding to a small molecule agonist or forming a heterodimer can induce a conformational change at the C-terminus of the FXR helix 11 at the dimer interface, thereby affecting the stability of the cofactor binding interface and the binding of the cofactor. The study also explored the allosteric regulation between FXR/RXR, providing new ideas for the development of new drugs in the future.
Liu Jinsong of the Guangzhou Institute of Biological Sciences Wang Na, a doctoral student jointly trained by the University of Science and Technology of China, is the first author of the article. The doctor of the Guangzhou Institute of Biological Sciences, Zhang Jiancun, has become a small molecule agonist in this study. The research was supported by the National Key Research and Development Program, the National Natural Science Foundation, the Guangdong Provincial Key Laboratory of Biomedical Computing, and the Natural Science Foundation of Guangdong Province.
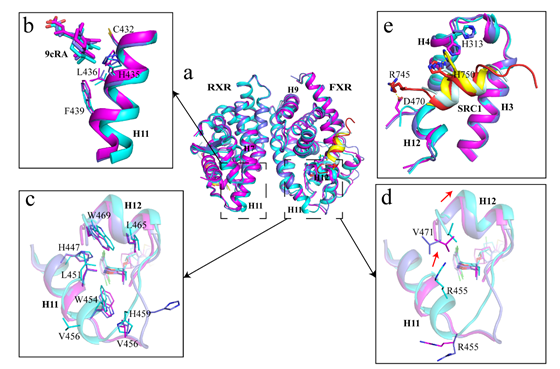
Conformation changes at the end of FXR/RXR heterodimer H11 caused by ligand
Preparation of Fenebute
1. Prepare the first intermediate
Benzaldehyde and ethyl acetoacetate into ethanol, stir at room temperature under the catalysis of organic alkali reaction with filter cake filtration after 45 ~ 50 h, after the completion of the filter with ethanol elution filter cake, dry cake solids, then ethanol filtrate concentrated to a quarter of the original volume, to cold, concentrate filtering alcohol washing, solid, merging two solid is the first intermediate; The mole ratio of benzaldehyde, ethyl acetoacetate and ethanol is 1:2.5 -3:12-15.
2. Prepare the second intermediate
The first intermediate is added to the mass concentration of 20% sodium hydroxide solution, and the reaction is stirred at 85-90°C for 2-5 hours. After filtration, the filter cake is used for filtration. After filtration, the filter cake is washed, the filtrate is combined and cooled, and the filtrate is cooled by stirring for 3h. Solid second intermediate; The mass ratio of the first intermediate to the sodium hydroxide solution is I: 1.8-2.0;
3. Prepare the third intermediate
The second intermediate was dissolved in pure acetic anhydride solution for reflux 3 ~ 5h. After the reflux, the acetic anhydride was concentrated and dissolved with toluene. After the dissolution, ammonia water was added, and the reaction took place at 60_65°C for 1 ~ 1.5 h. Filtrate, wash and dry the precipitated solid to get the solid third intermediate; The mass ratio of the second intermediate, pure acetic anhydride solution, toluene and ammonia water is 5:1-1.2:0.5-0.8:0.5-0.8;
4. Prepare Finebute
The third intermediate was dissolved in 25% sodium hydroxide solution and cooled to _5°C ~ 10°C. After cooling, sodium hypochlorite was added slowly within 15-20min for 1h reaction, followed by ice bath reaction of 0.5 ~ 1.5 h and water bath reaction of 0.5 ~ 1.5 h. Finally, the temperature was gradually increased to 60 ~ 80°C for 1-5 h, and the reaction was cooled again after completion. After cooling, hydrochloric acid was added in the ice water bath to adjust the PH to 2, and the decolorization was stirred at room temperature. After filtration, sodium hydroxide was added in the ice water bath to adjust the PH to 6-7, and the cooling was stirred for 2 hours. The mass ratio of the third intermediate, sodium hydroxide solution and sodium hypochlorite is 1.0:0.8-1.0:1.0-1.1.
Preparation of Fenebute
1. Prepare the first intermediate
Benzaldehyde and ethyl acetoacetate into ethanol, stir at room temperature under the catalysis of organic alkali reaction with filter cake filtration after 45 ~ 50 h, after the completion of the filter with ethanol elution filter cake, dry cake solids, then ethanol filtrate concentrated to a quarter of the original volume, to cold, concentrate filtering alcohol washing, solid, merging two solid is the first intermediate; The mole ratio of benzaldehyde, ethyl acetoacetate and ethanol is 1:2.5 -3:12-15.
2. Prepare the second intermediate
The first intermediate is added to the mass concentration of 20% sodium hydroxide solution, and the reaction is stirred at 85-90°C for 2-5 hours. After filtration, the filter cake is used for filtration. After filtration, the filter cake is washed, the filtrate is combined and cooled, and the filtrate is cooled by stirring for 3h. Solid second intermediate; The mass ratio of the first intermediate to the sodium hydroxide solution is I: 1.8-2.0;
3. Prepare the third intermediate
The second intermediate was dissolved in pure acetic anhydride solution for reflux 3 ~ 5h. After the reflux, the acetic anhydride was concentrated and dissolved with toluene. After the dissolution, ammonia water was added, and the reaction took place at 60_65°C for 1 ~ 1.5 h. Filtrate, wash and dry the precipitated solid to get the solid third intermediate; The mass ratio of the second intermediate, pure acetic anhydride solution, toluene and ammonia water is 5:1-1.2:0.5-0.8:0.5-0.8;
4. Prepare Finebute
The third intermediate was dissolved in 25% sodium hydroxide solution and cooled to _5°C ~ 10°C. After cooling, sodium hypochlorite was added slowly within 15-20min for 1h reaction, followed by ice bath reaction of 0.5 ~ 1.5 h and water bath reaction of 0.5 ~ 1.5 h. Finally, the temperature was gradually increased to 60 ~ 80°C for 1-5 h, and the reaction was cooled again after completion. After cooling, hydrochloric acid was added in the ice water bath to adjust the PH to 2, and the decolorization was stirred at room temperature. After filtration, sodium hydroxide was added in the ice water bath to adjust the PH to 6-7, and the cooling was stirred for 2 hours. The mass ratio of the third intermediate, sodium hydroxide solution and sodium hypochlorite is 1.0:0.8-1.0:1.0-1.1.
phenibut hcl powder,phenibut hcl vs faa, phenibut hcl capsules
Shaanxi YXchuang Biotechnology Co., Ltd , https://www.peptidenootropics.com