30 innovative medical products approved by the FDA in the first half of 2018: more than 60% are chronic disease monitoring equipment
In the first quarter and second quarter of 2018, the arterial network compiled 30 figures through screening of 510(k) pre-market notification devices for the FDA (US Food and Drug Administration) database from January to June. Medical products.
Compared with the product inventory reported by the previous arterial network in 2017 and 2016, the digital products for chronic diseases such as diabetes, cardiovascular and respiratory are still dominant.
However, in this year's database, the product heat for brain and neurological diseases such as stroke, epilepsy, and autism has gradually increased, indicating that in the new stage, the overseas market judges the trend of brain science. With the development of global aging, the market for diseases such as stroke and Alzheimer's disease that are biased towards the elderly will be further expanded, which has accelerated the process of R&D and application for such products.
Due to the wide variety of products approved by the FDA, involving drugs, equipment, consumables, etc., this article only discusses a part of the digital innovation involved in the trial product, in order to find some trends and laws.
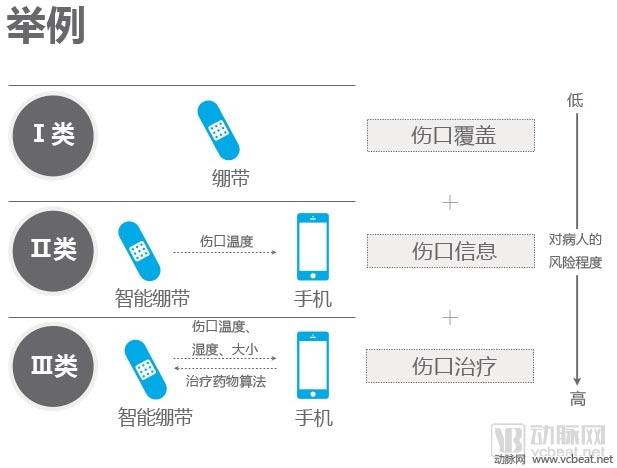
FDA-approved digital medical product classification principle
Before the Medical Equipment is reviewed by the CFDA, there are Class I, Class II, and Class III classifications. Before combing the product line, we can first look at the classification of the FDA. According to previous data from the Eggshell Institute, according to the FDA's identification, there are three types of mobile medical applications that need to be regulated:

Class I medical devices are medical device information that can link and control one or more medical devices, or display, store, analyze, and transmit specific patients, such as remotely displaying data monitored by patient bedside monitors, displaying brain waves, Control the sphygmomanometer cuff to inflate and deflate. Some of these types of applications may require only one 510(k) application, premarket notification before entering the market, and are simpler than other types of apps.
Class II medical devices are functions that can be converted into regulated mobile medical devices by means of Accessories, displays, sensors, or medical devices that are currently regulated by regulations, such as attaching mobile devices to blood glucose test strips. The App on the device is then used to check the blood glucose to make the mobile device a blood glucose meter. Almost all such medical devices are required to automatically submit a 510(k) application and make a pre-sales notice. Most of the digital medical products discussed in this article fall into this category.
Class III medical devices are stand-alone software that can perform analysis of specific patients and provide specific patient diagnosis or treatment recommendations for analyzing medical device data. FDA considers it to be an accessory to medical devices. Therefore, the classification it supervises will be the same as the classification of the highest risk equipment in various materials. This type of medical device must be approved by the FDA for premarket approval. During this period, clinical trials are required, and the time required for different products varies, several years or even longer, and the cost is high.
In the previous report, we also used bandages as an example to illustrate the difference between the three categories: ordinary bandages only cover the wound without other effects, then belong to class I; connected with the mobile phone, can view the wound temperature by application, and not Caution may also cause wound infection, there will be a slight risk to the consultation, belonging to category II; after connecting the mobile phone, not only the wound related information, but also the patient feedback, guidance, etc. through the drug algorithm, classified as Class III.
Main product information statistics
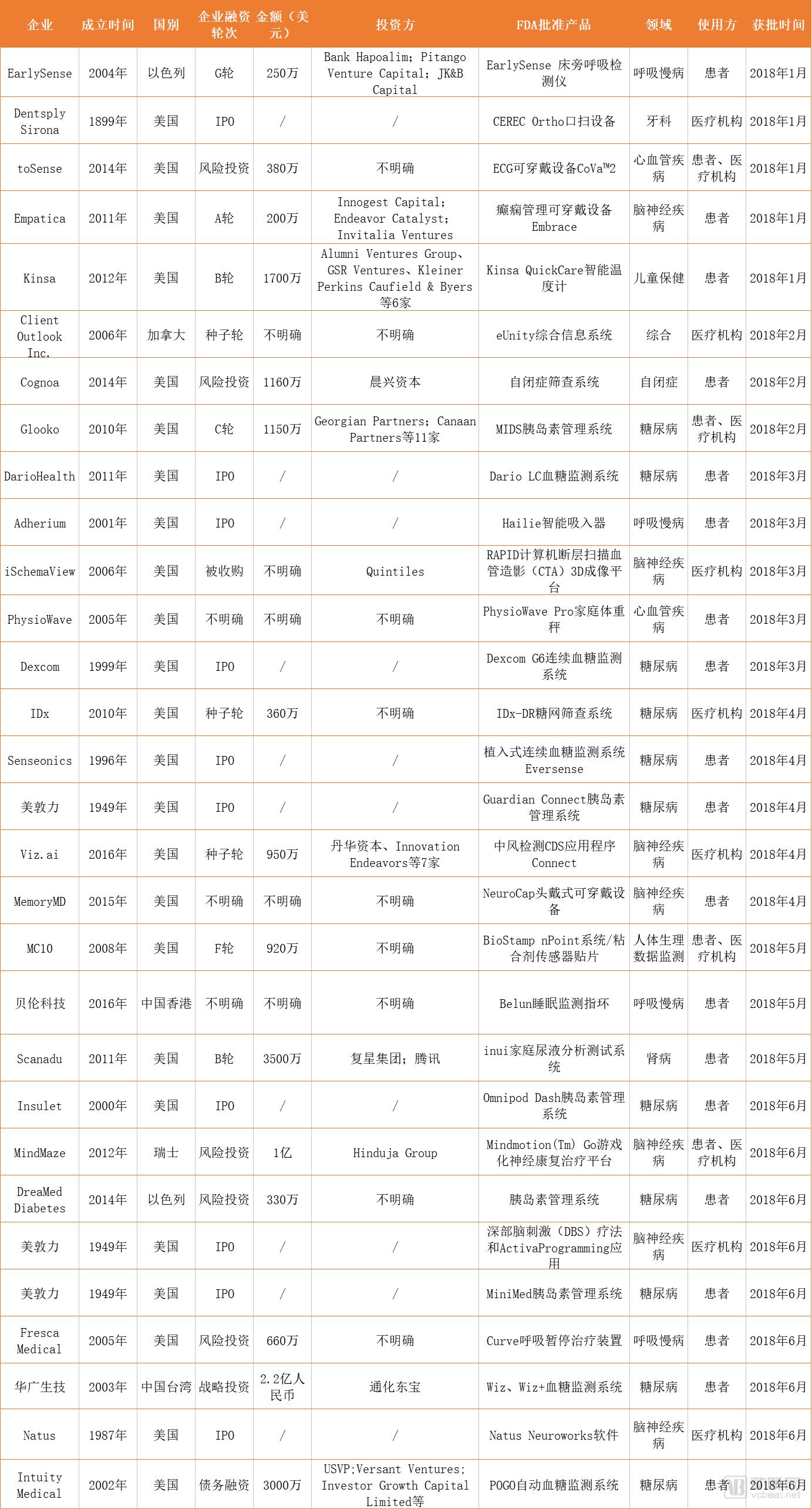
Note: DreamMed Diabetes and Viz. A is approved by the FDA's de novo, which is used for comparison products that are not legally listed in the United States, and that cannot be licensed through the 510(k) application route, to establish a special guarantee for the safety and effective assurance of the device. classification.
Figure 1 Data source: FDA official website, Crunchbase, arterial network
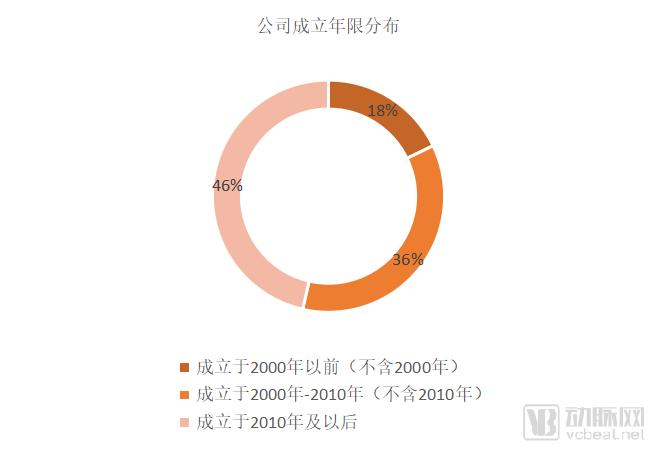
Figure 2 Product related to the company's establishment year distribution
In the development of the above 30 products (Medtronic's repeat products are only counted as one, a total of 27 companies), we classify them according to the age of establishment, in order to observe whether the innovative products are mainly developed by young startups. Although the statistics are only for reference, we can still see that 46% of the products were created after 2010, and 36% of the products came from companies established during the period of 2000-2010, only 18%, that is, 5 The company was founded in 2000, the 1990s, and is a relatively traditional medical equipment company with products and models.
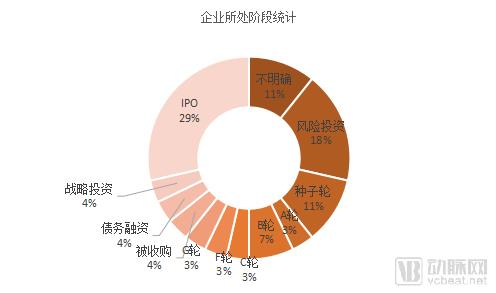
Figure 3 Distribution of the stage of the enterprise
From the perspective of financing rounds, enterprises that achieve IPO account for about 1/3 of the total, and the number is eight, including the old equipment manufacturers such as Medtronic, Dexcom, and Dentsply Sirona. Enterprises (including venture capital) accounted for 32%, concentrated in the B round of the G round of enterprises, 16%, strategic investment, debt investment and acquired companies accounted for 12%, each with one.
Therefore, from the perspective of R&D strength, companies in the late stage of IPO and strategic investment development have stronger R&D capabilities. After more in-depth exploration of the products of enterprises in the late stage of development through the FDA, we also found that This part of the company often has multiple categories for different diseases or different forms of product review, product iteration speed is faster.
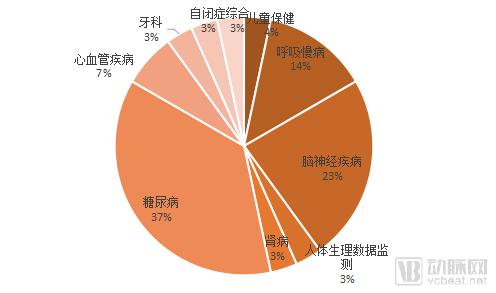
Figure 4 Product distribution in the field of disease
However, among the only four traditional companies, Medtronic has listed three products in the first half of 2018, targeting cranial nerve diseases and diabetes. Chronic diseases such as diabetes management have become the focus of research and development in many digital healthcare companies. Large medical device companies in the United States, such as Medtronic and DexCom, are increasingly investing in smartphone applications and consumer-grade connected devices.
In the first half of 2018, a total of 26 products were targeted at the monitoring or treatment of chronic diseases, which accounted for the absolute dominant position. Among them, the highest proportion was diabetes, with a proportion of 37%, followed by brain including stroke and epilepsy. In neurological diseases, respiratory diseases dominated by apnea are closely followed, and other chronic diseases include cardiovascular diseases and kidney diseases. The product form is based on wearable device/smart hardware + APP/support system.
In addition, there is no shortage of IDx-DR sugar mesh screening system, Viz. Ai's stroke detection program, Connect, and other products that use artificial intelligence innovation technology, are subject to numerous medical institutions.
From the current cutting-edge technology, brain health products are still the regular focus of the digital health field. Among the products approved by the FDA, the proportion of cranial nerve diseases accounted for 23%.
In the previous report of the Arterial Network, in March, Neurotrack's memory loss assessment and online prevention tools completed a $13.7 million financing for Series B financing. In June, Smart Brain Aging also announced the launch of SMART Brain, a virtual product for preventing Alzheimer's disease. U, while other industry players are also talking about the feasibility of similar digital methods in the cognitive field.
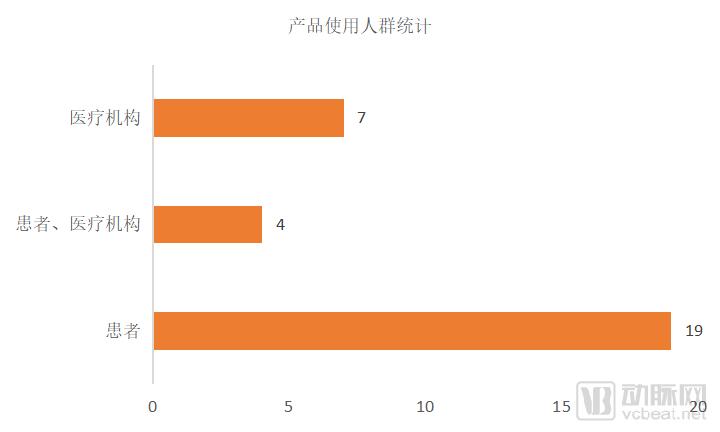
Figure 5 Main user statistics of the product
Among the 30 products, there are 23 patient-oriented products, accounting for 76.7%. Since some devices are not only targeted at a certain target market, there is a crossover in the statistics for the population. The business model is aimed at both C-side personal users and B-end enterprise users, mainly involving insulin management, ECG, EEG monitoring and other equipment for diabetes patients, helping individuals to stay at home or to be discharged from hospitals. Monitoring of indicators during the transition period from hospital to family.
In all kinds of diseases, the chronic disease market is undoubtedly the most suitable "hotbed" for home medical equipment. The chronic disease has a long disease period and requires long-term management. In the current medical environment, the diagnosis and treatment behavior is still mainly solved by the hospital. Management of chronic diseases requires time and effort if the patient is required to run the hospital again and again. Therefore, the concept of self-management emphasized in chronic diseases has spawned the birth and development of small home medical devices.
The FDA's digital medical products have always been the main developers in the United States, Israel and other countries that use advanced cutting-edge technology in medical innovation. Chinese companies have passed many FDA approvals on traditional equipment, and more companies are concentrated in Shenzhen. This is also related to the manufacturing atmosphere in Shenzhen.
Two innovative companies from China also appeared in this batch of FDA approvals. Among them, Huaguang Biotech was established in 2003 and is a leading enterprise engaged in biotechnology and medical testing systems in Taiwan. Currently focused on the development of self-glycemic monitoring systems for diabetes or professional medical institutions. Since 2012, Huaguang Biotech has passed 10 pre-market notifications of FDA 501(k).
diabetes
1 , Glooko's insulin management system

MIDS system (Source: mobihealthnews)
Glooko, a digital diabetes care company, was established in 2010 to obtain user-related blood glucose data indicators through a website or mobile app for unified recording, tracking and analysis. In February of this year, its mobile insulin dosage system (MIDS) has been approved by the FDA.
The platform can directly adjust the insulin dose using data collected from the patient's blood glucose meter. The MIDS module allows clinicians to create customized treatment plans and send them directly to patients with type 2 diabetes through the company's unified mobile diabetes management application.
At present, Glooko supports 26 kinds of blood glucose meters, covering 85% of the national brands, patients can eat 8 kinds of mobile devices to collect data, easy to integrate the scattered data collected by different devices.
Previously, Glooko, a diabetes data management platform, detailed two retrospective studies at the ADA meeting. The study showed that Glooko's mobile app reduced the average blood glucose level, glycosylated hemoglobin and hyperglycemia in diabetic patients. In the control experiment, the mobile phone application group performed more blood glucose tests than the control group in the same period of time. At the same time, the experimental results showed that the average blood glucose decline rate of the mobile phone application group was 3.54%, and the possibility of hyperglycemia was high. Reduced by 4.38%.
2. Medtronic's Guardians Connect Insulin Management System
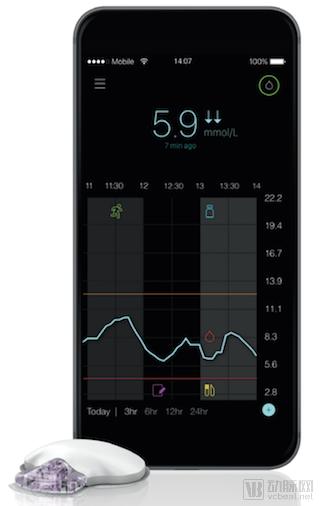
Guardian Connect intelligent continuous blood glucose monitoring system (Source: Medtronic official website)
Guardians Connect is the world's first intelligent continuous dynamic blood glucose monitoring (CGM) system for Medtronics for insulin-injected patients, displaying sensor blood glucose data, trends and alarms in an easy-to-use design. The Guardian Connect app is part of the CGM system and is independently connected to the smartphone and is not compatible with insulin pumps.
Consisting of Guardian's third-generation sensors and connected transmitters, the Guardian Connect system sends continuously collected blood glucose data via Bluetooth to the Guardian Connect app on the user's smartphone. The app can alert patients to hyperglycemia or hypoglycemia 60 minutes before a hyperglycemic or hypoglycemic event occurs.
In addition to adding convenience to the new sensor site, the company said in a statement that the average absolute relative difference (MARD) after calibration was 8.7%.
Guardian Connect system with Sugar. The IQ Diabetes Assistant works together to enhance the patient's understanding of daily blood glucose and the factors that affect them, giving patients a comprehensive understanding of diabetes.
3. IDX's AI Sugar Screen Screening System
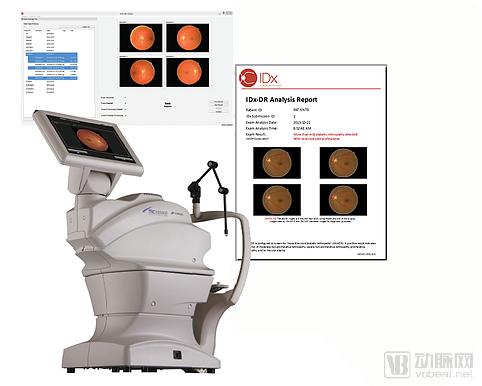
IDx-DR system (Source: IDX official website)
The FDA approved IDx's AI software system for autonomous detection of diabetic diabetic retinopathy in adults, known as IDx-DR. According to the FDA, this decision marks the first artificial intelligence-based diagnostic system authorized by the US Food and Drug Administration to commercialize in the United States, which can provide screening decisions without the intervention of a clinician.
The IDx-DR algorithm analyzes the images taken with the Topcon NW400 retinal camera and uploads them to the cloud server. Within a few minutes, the software provides a flat result for the doctor to detect if the patient has diabetic retinopathy. It is worth noting that since the results of the system do not require the interpretation of a clinician, they can be provided to a clinic that does not have an ophthalmic service.
The FDA approved the software based on clinical data from 900 retinal images of diabetic patients collected from 10 primary care sites. Among the 900 patients with diabetes, IDx-DR was able to correctly identify mild diabetic retinopathy at 87.4%. At the same time, it was correctly identified at a time of shortening by 89.5%.
4. versonics' Eversense CGM system
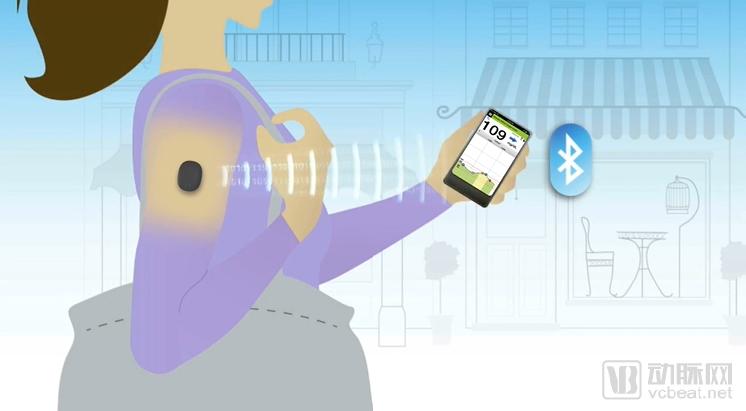
Schwery CGM system schematic (Source: Senseonics official website)
Senseonics' positioning of the Eversense CGM system is intended to be the first and only CGM with an implantable sensor that can last up to three months. This is a device that monitors a patient's blood glucose concentration by implanting the upper arm of a diabetic patient, continuously monitoring blood glucose through an implantable sensor, a detachable and rechargeable smart transmitter, and a convenient smartphone application.
The device contains a fluorescent polymer that is sensitive to blood glucose levels. When the blood glucose concentration changes, the signal transmitted by the material changes and is transmitted to the mobile device worn by the patient in real time.
The transmitter can also connect to the user's computer via USB to upload blood glucose history data. If the concentration is too high or too low, the device will alarm. But this system requires twice-a-day fingertip blood sampling, and Senseonics hopes to improve the defect and remove the blood collection to make the system more convenient.
5, Dario LC blood glucose monitoring system

Dario Health Blood Glucose Monitoring System (Source: Dario Health's official website)
Dario's systems include pocket devices, blood glucose meters, disposable test strips, blood collection devices and companion smartphone applications.
Dario Health's approval was based on the iPhone's removal of the 3.5mm headphone jack. The Dario blood glucose measuring device originally transmitted blood glucose readings to the Dario Smart Diabetes on the mobile device via the 3.5mm headphone jack of the smart mobile device. Management (Smart Diabetes Management) App.
Therefore, Dario users will also be troubled when they decide to upgrade their new iPhone models. After Apple replaced the 3.5mm headphone jack with Lightning interface, DarioHealth also followed the market to iterate. The new Lightning interface blood glucose measuring device can fully support the old and new iPhones, providing the same quality user experience. The blood glucose monitoring solution includes a blood glucose meter, a disposable test strip box that holds 25 test strips, and a blood collection unit.
The Dario Blood Glucose Monitoring System synchronizes with the Dario Smart Diabetes Management App to measure, record and track real-time blood glucose values, monitoring, tracking and managing diabetes anytime, anywhere. DarioHealth also provides an index of 500,000 foods to calculate carbohydrate intake.
Cardiovascular field
1. ToSense CoVa2 ECG Monitoring System

CoVa2 ECG monitoring wearable device (Source: mobihealthnews)
ToSense's CoVa2 is a wearable device for remote monitoring of individual vital signs and electrocardiograms. It is worn around the patient's neck and is connected to the chest with two disposable electrodes to monitor human data.
The product received the FDA's second 510k license in the second quarter of the FDA. The new license will allow the company to update the software and add new features to measure patient stroke volume, heart rate, pleural effusion, heart rate variability, respiration, skin temperature and other metrics while allowing devices to connect to mobile applications, allowing Medical professionals remotely view measurement data and single-lead ECG waveforms.
The application scenarios include remote monitoring for doctors, monitoring of patients during the transition from hospital to home after discharge, and monitoring of patients during clinic visits. Clinical professionals can quickly diagnose, classify and formulate data based on patients. The patient's treatment plan.
2. Stanford University Incubation of PhysioWave Project
Developed by Stanford University's PhysioWave, the smart weight scale is FDA-approved for its Pulse Wave Velocity (PWV) Cardiovascular Analyzer Scale.
Pulse wave velocity (PWV) refers to the pressure wave velocity transmitted along the wall of the aorta generated by the heart every beat pulse. It is a simple, effective and economical non-invasive index for assessing arterial stiffness. It can comprehensively reflect various dangers. Factors that damage blood vessels are independent predictors of cardiovascular events. The pulse wave velocity can reflect the elastic state of the large and middle arterial system, and it is non-invasive, simple, effective and reproducible, and can reflect the real-time changes of arterial function.
The scale is used to measure the hardness of blood vessels that are transported from the heart to the body. Therefore, the company claims that its technology can identify patients at high risk of having a cardiovascular disease. In addition to PWV analysis, this new scale called PhysioWave Pro measures pulse, weight and BMI, and uses a dedicated tablet as a user interface.
PhysioWave was the first company to receive FDA-approved PWV functionality for a home scale, but it was not the first company to promote the feature. Earlier this year, Nokia cancelled PWV functionality on its Body Cardio scale due to concerns about FDA's potential regulations.
Neurological disease
1. Empatia's Embrace Epilepsy Detection Wearable Device
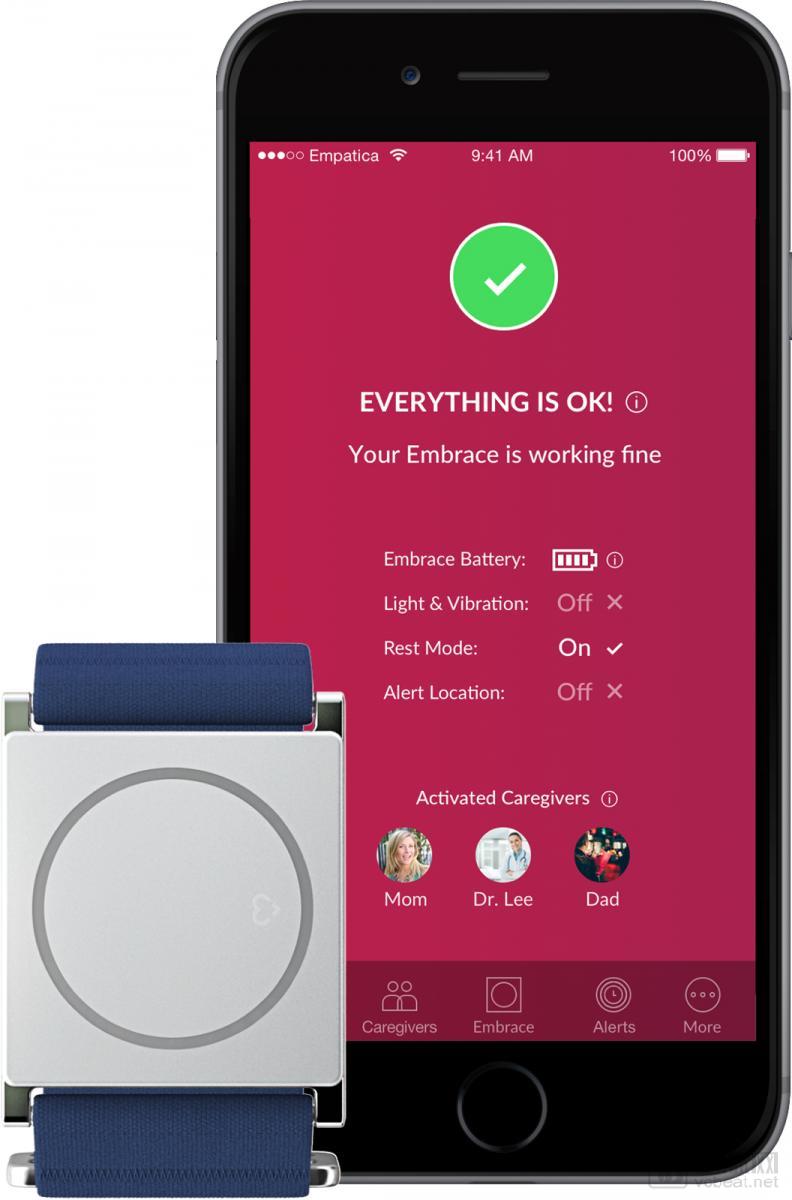
Embrace equipment and software (Source: mobihealthnews)
Empracea's Embrace is a consumer-oriented seizure detection wearable, which received an FDA's 510(k) license in January 2018. Currently, the wearable device has been used in clinical trials by the pharmaceutical company Sunovion and is used as a prescription rather than an over-the-counter device, which means that the user must obtain a prescription from their neurologist to use the device's seizure detection function.
2. iSchemaView's RAPID CTA 3D imaging platform
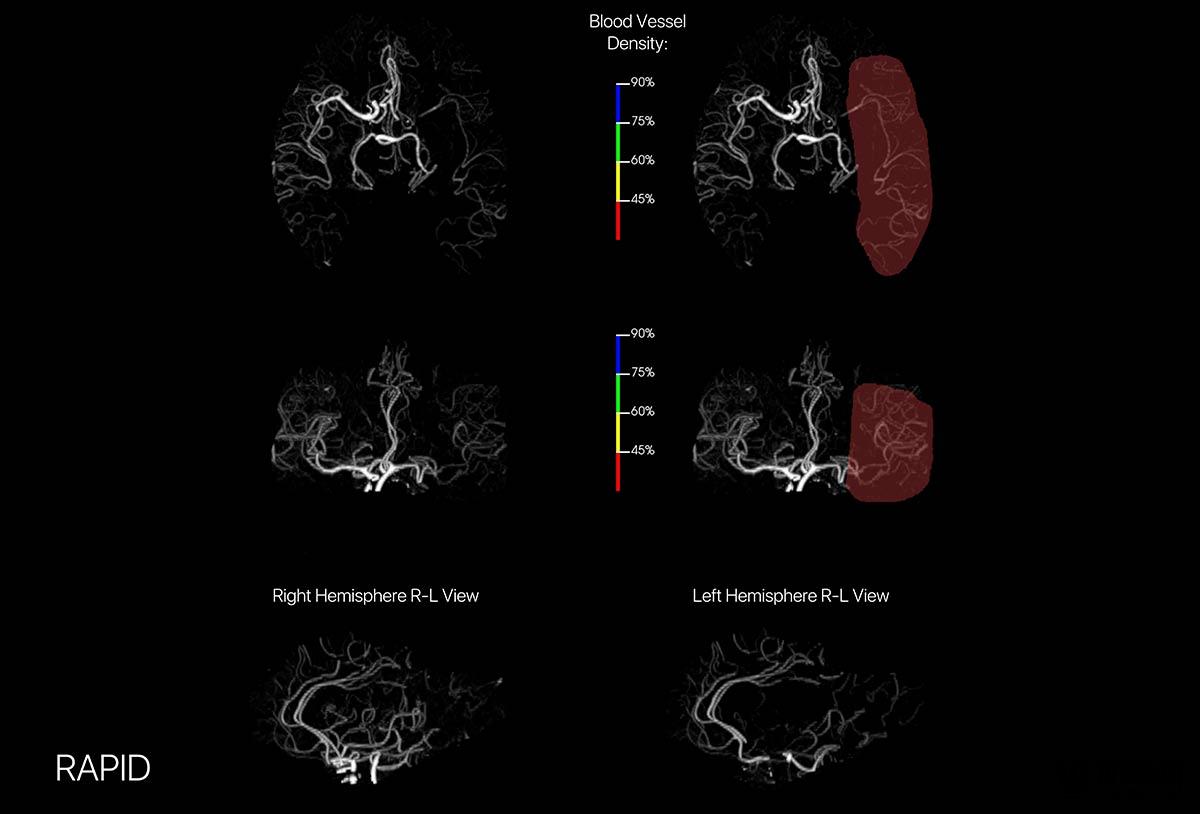
RAPID CTA image (Source: iSchemaView official website)
iSchemaView Imaging has received FDA's latest product, RAPID CTA, a 3D imaging platform for computed tomography angiography (CTA). According to the company, CTA scans are used to help clinicians observe the cerebral arteries in patients with thrombosis.
Based on clinically validated machine learning algorithms, RAPID ASPECTS automatically generates standardized scores that allow doctors to easily understand the extent of ischemic changes in patients and determine the feasibility of thrombectomy (clot removal).
In addition, RAPID ASPECTS provides clear brain visualization so clinicians can better examine each area and confirm automatic scoring.
This technology allows doctors to fully understand the blood vessels in the brain, and doctors can rotate images at different angles on the platform. The CT map also has four colored overlays so doctors and caregivers can see which areas of the brain have reduced blood vessel density.
RAPID CTA image maps are immediately available to physicians via email for viewing on any device, PACS system and web browser. These images provide an intuitive and easy-to-interpret cerebrovascular view that helps doctors make clinical decisions, triages, community hospitals and specialists, and patient referrals.
There are currently more than 350 stroke centers in the world using RAPID. These centers produce more than 50,000 scans per day, and more than 200 systems are expected to be operational in 2018. The estimated scan count is close to 100,000 per year.
3. MindMation's gamified neurological rehabilitation platform MindMotion Go
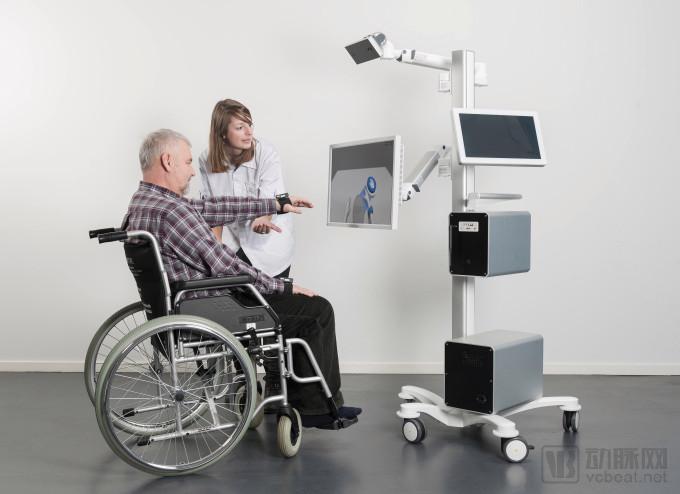
MindMaze product indication (Source: techcrunch)
In June 2018, MindMotion Go, based in Switzerland's MindMaze, received FDA approval as a gamified neurorehabilitation treatment platform for home rehabilitation of patients with moderate and mild brain damage.
Using Microsoft Kinect-based motion capture technology, MindMotion Go provides patients with a range of activities that are set in a virtual reality environment that promotes patient motion and functional recovery through VR technology in individual gamification scenarios.
Slow breathing
1. Hailie smart inhaler for chronic obstructive pulmonary disease
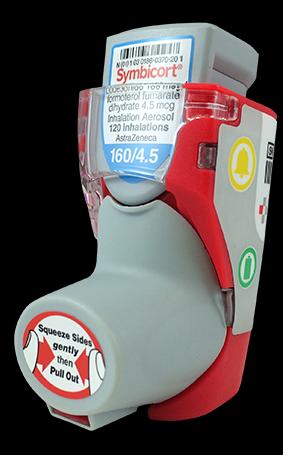
Adherium smart inhaler (Source: Adherium official website)
In March 2018, the intelligent inhaler company Adherium received a 510(k) license, which was sold on the counter of the inhaler monitoring device along with AstraZeneca's Symbicort aerosol inhaler (called SmartTouch for Symbicort).
Adherium manufactures a software device that is ported to a patient's inhaler as part of a self-management program to monitor and encourage medication adherence. It is designed for easy installation and removal, and includes three buttons to help patients access viewing reminders, battery monitoring and Bluetooth low energy pairing.
The inhaler can also help the clinician to ascertain the patient's record of using the inhaler, such as when and how the patient misused the inhaler, either by inverting the product or using the inhaler at an inappropriate time, and monitoring the device through the device. Avoid the use of drugs.
2, sleep apnea monitoring products Earlysense

EarlySense Sensors and Software (Source: EarlySense official website)
In 2015, EarlySense was named one of the “Fierce 15†medical technology companies by Fierce Medical Device, positioning it as the most promising private medical technology company in the industry; in 2016, Early Sense was named the fastest growth of Deloitte technology in 2016. One of the top 50 companies; in 2017, EarlySense was named one of the world's most innovative companies by Fast Company.
The EarlySense system is a fully integrated, integrated patient monitoring platform that provides continuous vital signs and motion information monitoring for general care patients with the goal of improving patient safety and minimizing false positives. The entire system consists of four parts: sensor, bedside monitor, data transfer system and EMR integrated system.
3. Wearable smart “rings†developed by Belem Technology

Belem Smart Ring (Source: Belem Technology Website)
Belun Technology is the second developer of digital medical products approved in the first half of the year. The company mainly provides medical-grade lightweight wearable solutions, the main product of which is a ring-type intelligent hardware. Simultaneous measurement of beat-by-beat heart rate, pulse oximetry and exercise at night can be performed without disturbing the user's sleep. Physicians can rely on data collected by the ring and analyze it through Belém's cloud computing platform to diagnose and monitor post-treatment effectiveness of obstructive sleep apnea.
Belun PHA (Personal Health Assistant) can be used to measure bronchial and central aortic blood pressure and to assess arterial stiffness index (ASI). Users can effectively and effectively monitor cardiovascular health in their homes conveniently and economically. Preventive care and early treatment can avoid accidents caused by apnea and reduce medical costs.
Summary: Which developers are keen to apply for FDA?
The above-mentioned applications for the approval of the FDA for digital medical products in the first half of 2018 reflect the following trends:
1. The number of startups that have been established for less than 10 years is the largest, with 13 in total. This also shows that Internet medical startups are stepping up to break through regulatory barriers. Digital medical products of the same kind of disease are usually varied, and a very important factor in obtaining user trust is whether it is approved by the FDA and can be directly applied to the clinic.
2. Large enterprises with relatively mature IPO business models will continue to expand their products under the premise that products have passed the FDA in the early stage, and even involve different disease areas, constantly iterating products, and continuously making breakthroughs in innovation.
3. Previously, domestic products passed the FDA, which is usually traditional equipment for equipment and consumables. However, in terms of innovation, a small number of domestic companies have emerged to explore innovative solutions in the field of chronic diseases. Such equipment is usually targeted at large populations. Diseases such as diabetes, respiratory, sleep disorders, etc.
4. Patient-oriented self-management programs are increasingly appearing on the market, and the main direction is chronic disease products, not only because of the accessibility of medical services, but also because chronic diseases are conditional and suitable for self-management. If the patient himself will take insulin, it will be a great subversion for doctors and medical institutions. The traditional human resources are being replaced by standardized equipment or processes.
Foley Catheter,bardex foley catheter,silicone foley catheter,catheter foley,latex foley catheter
Anesthesia Medical Co., Ltd. , https://www.jssinoanesthesia.com