How can we innovate domestic medical equipment to polish national brands?
With the deepening of the medical system reform, the improvement of people's health awareness and the aging of the population, the medical device market is continuously releasing demand. However, another reality is that, in recent years, foreign companies have rapidly monopolized 70% of the market by continuously acquiring local enterprises, which has directly caused the high cost of medical institutions and high maintenance costs. Brought price barriers.
"In recent years, although the level of innovation in domestic medical devices has improved, the original innovation capability is still insufficient. This is the biggest bottleneck restricting the development of domestic medical devices." In the view of Fang Ming, chairman of Zhejiang Kehui Medical Devices Co., Ltd., I want to think about it. Breaking the situation that high-end medical devices are basically imported, domestic medical device companies must make innovative articles.
The status quo of foreign capital monopoly needs to be broken
Medical devices are both mysterious and strange to ordinary people. In fact, with the development of society, medical devices are closely related to everyone. Under the new economic normal, the medical device industry is regarded as a green growth point of “to be developedâ€.
However, an unavoidable reality is that the Chinese medical device market, the fat “Nuggetsâ€, is currently being held by multinational giants. With industry-leading advantages, first-mover advantage and technological innovation, foreign-funded enterprises occupy the commanding heights of R&D, production, branding and marketing of high-end medical devices.
The long-term monopoly of multinational medical equipment companies not only directly causes the high cost of medical institutions to purchase, but also maintains high maintenance costs. It also poses price barriers for patients to see and check.
In order to promote the localization of medical devices and alleviate the problem of “expensive medical treatment†for the people, on May 9, the General Office of the State Council issued a notice on the reform of the medical and health system, clearly strengthening the investment in new drug creation and localization of medical devices, and researching and promoting the domestic production of medical devices. The policy measures, which undoubtedly released a positive signal.
However, the pace of localization of medical devices is not a thousand miles. In Fang Ming's view, there is still a big gap between national brands and foreign countries. "Since most of the basic research is carried out by universities, the disconnection between technology and industrial production has become a common problem, which has led to the lack of original technology and core technology of national enterprises. The level of research and development is not high."
In addition, Fang Ming also revealed that domestic medical device companies generally face the same flaw: insufficient talent reserves. The medical implant industry involves many technical fields such as information, materials, precision machinery, life sciences, etc., and there are many kinds of products. The technical differences involved are large, and it is often difficult for enterprises to find the counterparts. "No talent, innovation and What do you talk about?"
The predecessor of Kehui Medical Devices Co., Ltd. is the Jinhua Computer Research Institute, which has been in the international medical device market for more than 20 years. Although the transition to the country is only five years, compared with other entrepreneurs, Fang Ming, who was born in research, has a more keen judgment and scientific understanding of the industry.
“After the status quo, it will gradually be eliminated by the market.†Fang Ming believes that it is a prerequisite to successfully implement localization of equipment and break the monopoly of foreign capital, and its effective means should be to strengthen the cooperation of production, learning, research and medicine to help the country. Enterprises have mastered more product core technologies.
Industry, study and research "crowd group" development allows technology to serve health
Not only is Fang Ming, but now more and more domestic companies are fully aware that only by strengthening innovation can they gain a living space in the future market.
At present, domestic conventional medical devices have basically achieved independent production, and high-end medical devices have also been involved, but the situation dominated by low-tech, low-tech low-end products has not changed.
“In the face of this market, can we do something?†It is painful that domestic excellent medical device companies are frequently acquired by foreign investors. Fang Ming has been trying to “win†the market initiative. However, he also knows that this is only one The power is far from enough. We must seek a "aggregation point" to instigate the entire industry and break the monopoly of foreign investment.
After many years of planning, on the 17th, the National Medical Implant Innovation Technology Alliance, established by Zhejiang Kehui as a support unit, was established in Hangzhou, Zhejiang Province. The alliance has gathered 30 domestic and foreign enterprises and research institutes in the field of implant medical devices. Institutes, institutions of higher learning, medical institutions.
For Fang Ming, this is the "fulcrum" of the break.
Breathing Filter- Heat & Moisture exchange filter(HMEF)
Disposable breathing system filters are intended to reduce the transmission of microbes and other particulate matter in anaesthesia and intensive care unit.
The HME filters addtionally provide the function of warming and humidifying the inspired gas by conserving the exhaled heat and moisture.
The filter can also used in spirometry to protect the spirometer from expired infective droplets.
The use of our filter at patient end can effectively reduce the risk of cross-infection during anaesthesia and intensive care and will protect the anaesthesia and breathing equipment.
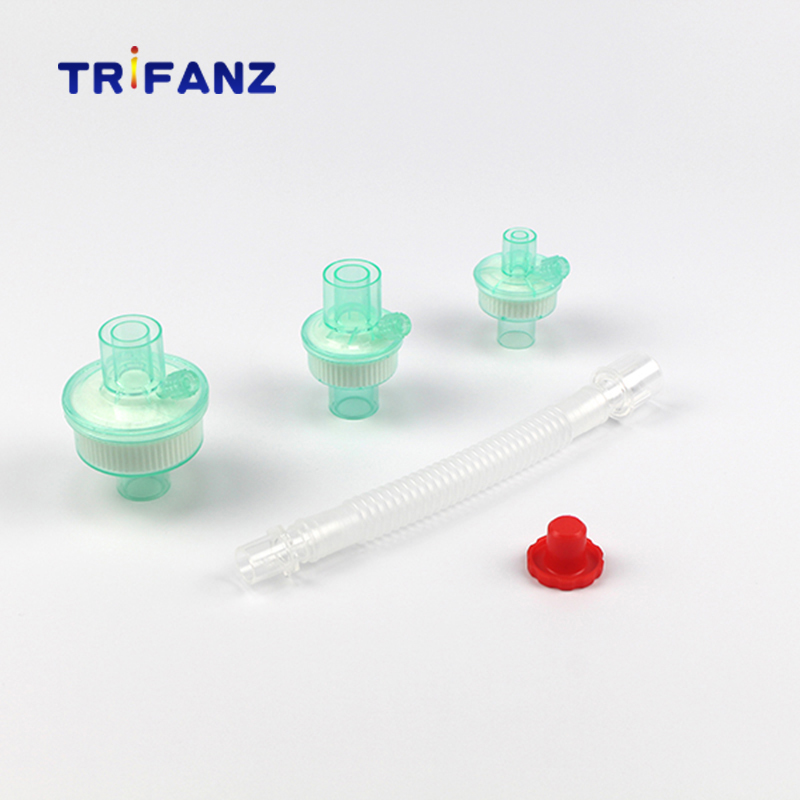
Features&Benefit
Ø Designed in light weight,enhancing patients comfortable.
Ø Gas sampling ports available with tethered cap or luer cap.
Ø The shell is made of medical grade ABS or PP or K-resin with high quality and non-toxic.
Ø Adopt imported 3-layer filtering medium with high efficiency ultrasonic sealing process.The filters have compact appearance and reach the international quality standard.
Ø With advantages of good sealing,low breathing flow resistance and small dead space.
Ø Connector:15mm/22mm.
Ø prevent particles, bacteria and other pathogens in anesthesia and breathing circuit from entering the respiratory system.
Moisture Exchange Filter,Pp Paper Material Hme,Surgical Breathing Filter,Disposable Hme Filter
Hangzhou Trifanz Medical Device Co., Ltd , https://www.cfzmeds.com