Medical equipment is difficult to take off: three barriers and three obstacles to interpret
Recently, the state has issued a good policy on medical devices: In recent years, the state will focus on supporting 10 to 15 large-scale medical device enterprise groups, supporting 40 to 50 innovative high-tech enterprises, and establishing 8 to 10 medical device technology industrial bases. 10 national-level demonstration and application bases for innovative medical device products.
The National Development and Reform Commission, the Ministry of Industry and Information Technology, the Ministry of Finance and the Health Planning Commission are jointly drafting a special project for industrial revitalization and technological transformation. The total special support fund is 1.5 billion yuan. Among them, the development of high-performance medical diagnosis and treatment equipment in 2013 will focus on medical imaging equipment, in vitro diagnostic products, therapeutic equipment and other fields to achieve coordinated development of the upstream and downstream of the industrial chain.
In the face of the current situation of China's large-scale medical equipment market being monopolized by foreign capital, the relevant leaders said that China will vigorously develop domestic medical equipment in the future, reduce medical costs, and strictly implement the government procurement law to ensure that financial funds preferentially purchase domestic medical equipment. Leading technology with independent intellectual property rights in China gives a tilt when it comes to medical insurance.
However, at present, China's national medical device industry is highly polarized. At present, there are 60 listed companies in China and abroad that are doing routine equipment, making diagnostic products, and doing home medical products. Such as Shenzhen Mindray, China Resources Wandong, Shanghai Kehua, Jiangsu Yuyue and so on. Companies with domestic original and global leading technologies such as stereotactic ultrasound focusing therapy system, gynecological radiofrequency ablation technology, excimer laser human eye aberration correction system, body rotation gamma knife, and sleep monitoring system are not listed. Isn't there an annual sales that can achieve over 100 million? Why is this happening? Because of the domestic original, the world's leading technology companies face three thresholds at the policy level: product registration certificate, price or bidding, medical insurance. There are three major obstacles in the development of the enterprise, namely, the capital, the boss's mentality, character, mind and the way of employing people.
Threshold 1: Product Registration Certificate
First, a medical device must obtain the corresponding qualification before entering the market, that is, obtain the product registration certificate, otherwise the product cannot be sold in the market. At present, the state divides medical devices into three categories. One type of medical device product registration certificate is set up by the Municipal Food and Drug Administration, the second type medical device product registration certificate is set up by the Provincial Food and Drug Administration, and the three types of medical device product registration certificates are in the State Food and Drug Administration. Office, the three types of medical device product registration certificate is the most difficult to take, enterprises usually take four or five years, or even longer. Therefore, in the society, there is a company that specializes in the certification of the three types of medical device product registration certificates for enterprises. It is in the context of the difficulty of the certification:
The State Food and Drug Administration issued a notice on June 26, 2013, deciding to delegate the responsibility for the examination and approval of the change of some of the three types of medical device registration certificates undertaken by the Bureau to the provincial food and drug regulatory authorities, and to lower the capital into the medical devices. The threshold of the industry further simplifies the process of government affairs.
Recently, the drug regulatory department will soon introduce two new regulations for medical device supervision, which will guide the registration of innovative medical device products and further simplify the requirements for re-registration of medical devices. According to industry insiders, the introduction of relevant measures will help promote the integration of medical device industry and make it more conducive to the development of innovative medical device companies.
Threshold 2: price or tender
It is only the first step for a company to get a product registration certificate. What is more important is that it must be handled at a price. There is no way to charge the hospital without price, how long does it take to run the national price? The answer is more than 10 years. For example, China's original technology, with the leading technology of gynecological radiofrequency ablation technology in the world, took ten years to set prices in five provinces. The prices of the provinces vary from province to province, but at least one year or so. Some provinces only have to set prices for three years, such as Zhejiang. The prices of the enterprises need to be submitted by two or three top three hospitals in the province. After the expert argumentation and price arguments, they will be tested. The price of the edition can only be converted into a formal price after one year. If you want to apply for national prices, you must have already processed the price in more than 5 provinces. These undoubtedly increase the difficulty for manufacturers to set prices, Beijing, Guangdong, Guangdong, Zhejiang, the price of the office is very difficult.
Single Packed Mottled Waxy Corn
Waxy corn comes in a variety of colours. Some people wonder if waxy corn is a genetically modified product. In fact, it is not. Waxy corn originated in China. It is caused by a genetic mutation. Artificial selection gradually led to the emergence of a type of tannin.
Waxy corn, also known as waxy corn, is sticky corn. The grain has coarse, waxy endosperm, similar to shiny, glassy (clear) grains such as hard and dented corn. Its chemical and physical characteristics are controlled by a recessive gene (wx), which is located on chromosome 9. 100% of the starch in the endosperm is straight-chain starch.
Coloured glutinous corn is generally white, yellow, red, purple and black, with white, yellow and purple corn being the basic colours. Purple and white hybrids naturally become purple if the purple gene "beats" the white gene and vice versa, so if the two tie we see white and purple corn. Purple can turn into red and black corn, or as we often say, "red is purple and black is purple". Of these colourful corn, the most common yellow waxy corn is the most nutritious as it is rich in carotenoids...
Currently, the only genetically modified foods sold on the Chinese market are soybean oil and papaya. Waxy maize is a hybrid variety and is not associated with genetic modification. Therefore, it can be concluded that glutinous maize is a hybrid variety and has nothing to do with genetic modification.
Genetic modification is a type of "genetic engineering" in modern science and technology, which makes use of modern molecular biology techniques. Hybridisation is the mating of individuals of different genotypes to produce offspring that are different from the original "pure" breed. In a sense, it belongs to the natural exchange of genes that can occur in nature.


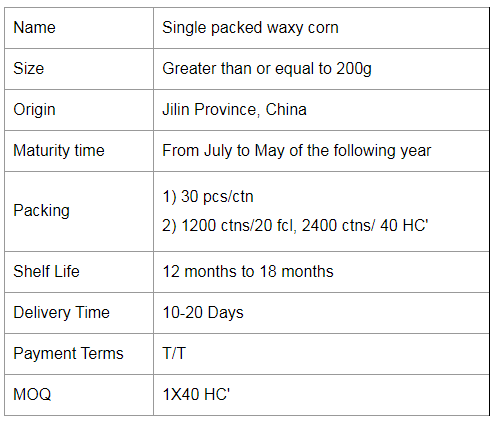
Colorful Waxy Corn,Colorful Mottled Waxy Corn,Single Packed Mottled Waxy Corn,Single Packed Colorful Waxy Corn
Jilin Province Argricultural Sister-in-law Food Co., Ltd. , https://www.nongsaocorns.com