Thermo Retention Time Alignment software function introduction
Overview
The Thermo Retention Time Alignment software guarantees the TRACE1300 Series GC for better analytical quality in less time and with less effort. It eliminates retention time deviations caused by changes in the chromatogram and allows for retention times between different systems and between different injections. This software will match the retention time obtained in any TRACE1300 Series GC system to other system retention times using the same type of column. A pesticide database with this software function has been provided, which contains retention times of 670 pesticides under locked conditions. This software can be used with any GC detector of the TRACE1300 Series GC (including triple quadrupole mass spectrometry), any inlet or injector type, capillary column of different lengths with the same stationary phase and inner diameter.
The process of using a software is as follows:
1 Select the appropriate normal paraffin (C10H24 n-decane is recommended)
2 Set the appropriate oven thermostat program so that the retention time of the above selected n-alkanes is 10 < RT < 20 min;
3 Record the RT (retention time retention time) of the normal paraffin;
4 After the column is cut, run the normal paraffin again using the same constant temperature program, and record its RT and air peak time (Void time);
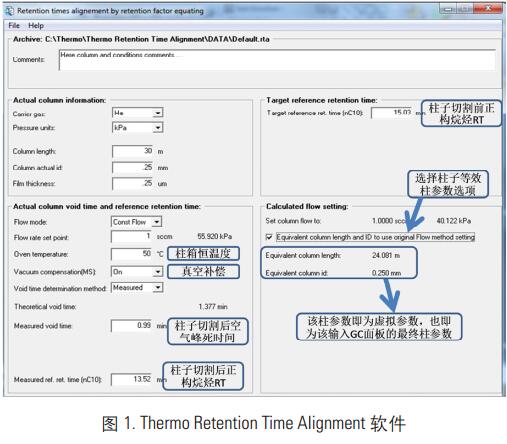
Enter the above parameters into the RT calibration software (see Figure 1) to calculate the final column dummy parameters and enter them into the GC panel.
Two example analysis instrument method:
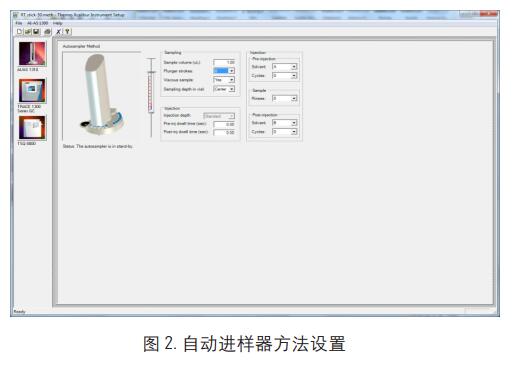
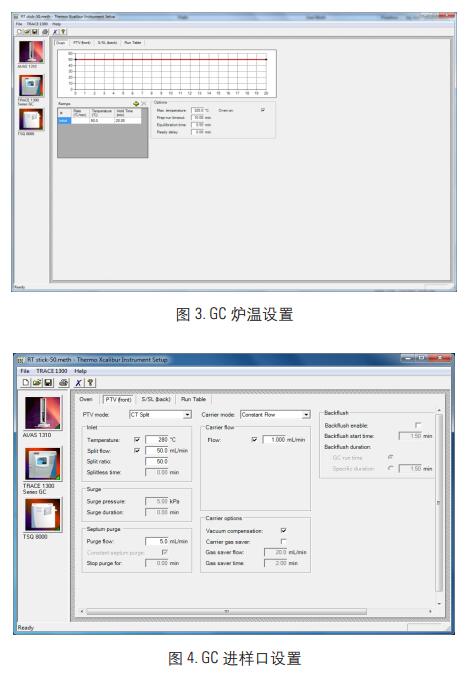
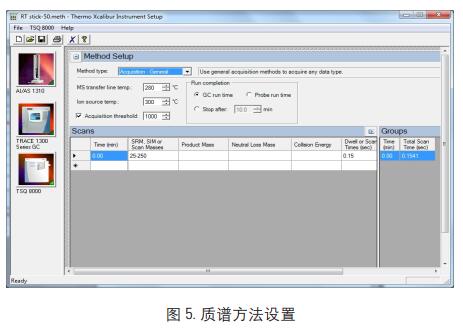
1. GC method settings (Figure 2, Figure 3, Figure 4)
Oven: 50 ° C (maintained for 20 min)
Inlet temperature: PTV CT split injection, split ratio 50: 1, column flow rate 1.0ml/min
Transmission line temperature: 280 °C; Injection volume: 1ul
2. Mass spectrometry method (Figure 5)
3. Experimental results According to the above instrument method, data collection was performed on n-decane standard products. The data results are shown in Figure 6:
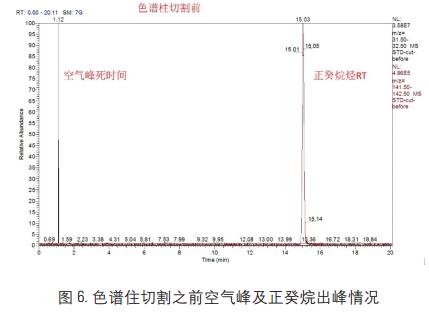
After the column is cut, the n-decane pure product is subjected to secondary data acquisition while the GC conditions remain unchanged. The data results are shown in Figure 7:
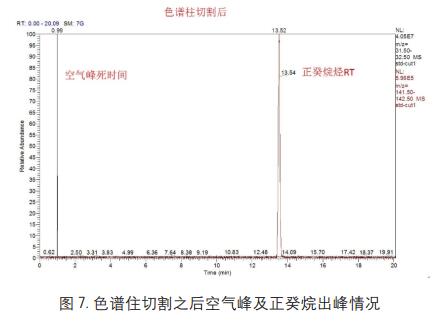
The two sets of data obtained by the above operations are substituted into the software, and the relevant information after the column cutting is automatically calculated by the software, and the calculation result is as shown in FIG. 8:
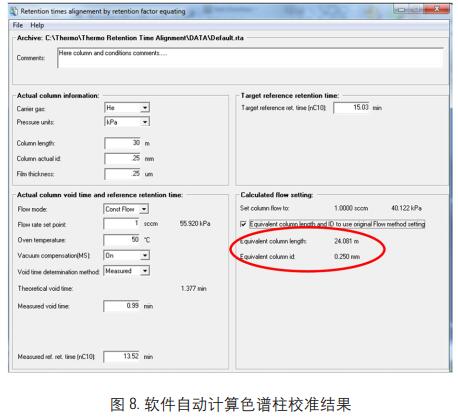
The above software calculation results are entered into the GC system through the GC column setup software, Figure 9, and the retention time correction of the compound is performed.
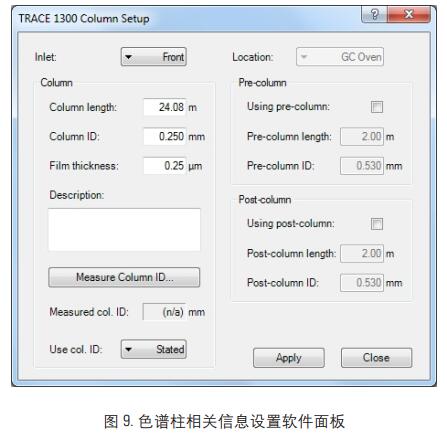
After the retention time calibration, the third data acquisition was performed on the n-decane standard. The data results are shown in Figure 10:
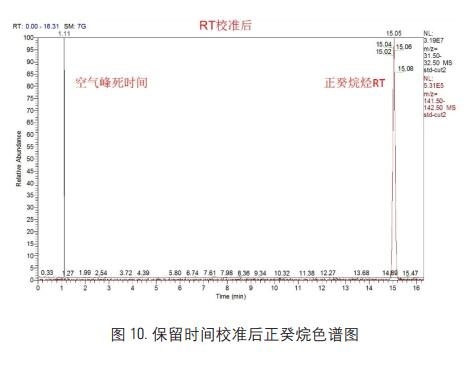
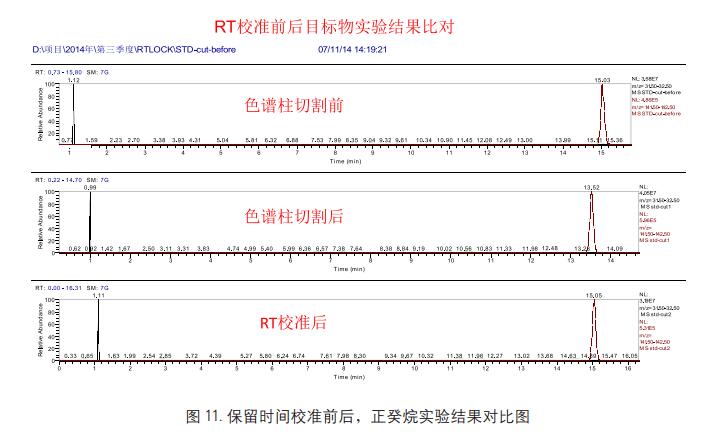
Using the Thermo Retention Time Alignment software, we were able to accurately calibrate the retention time of n-decane, and the experimental comparison of the target retention time before and after calibration is shown in Figure 11:
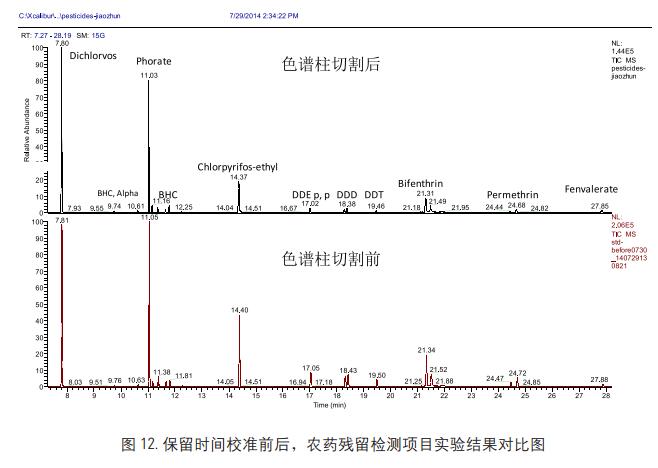
Application analysis of pesticide residue detection examples, the pesticide varieties involved are organic phosphorus, organochlorine and pyrethroid pesticides. We use Thermo Retention Time
The Alignment software allows the target compound to remain unchanged after column head cutting maintenance, ensuring stable data processing methods and improving the efficiency of multi-residue detection and analysis.
summary
Retention time calibration of compounds using Thermo Retention Time Alignment software can greatly improve laboratory productivity, especially when performing multiple pesticide detection, not only optimizing the experimental procedure, but also allowing retention times of target and unknown compounds. Can be reproduced, so that we can directly use the retention time for qualitative analysis. Thermo Fisher has now developed a pesticide screening method and established a retention time database of 670 pesticides. By using the ThermoRetention Time Alignment software, you can make the pesticide retention time on your chromatograph consistent with the value of the database. The database is searched by computer to achieve the qualitative results of compound screening, which is of great significance for the screening of unknown compounds.
Push&Turn Cap Vial, Push&Turn Cap Tube, Plastic Push&Turn Cap Vial, Click Vial, Children Resistant Vial, Child Resistant Plastic Vials, Child Proof Cap Vial
Push&Turn Cap Vial,Click Vial, Children Resistant Vial, Child Resistant Plastic Vials
Luck Medical Consumables Co.,LIMITED , https://www.luckmedical.com