NAT COMMUN | Proteomics (TMT+label-free) Deciphering Aging Passwords
NAT COMMUN | What is the degradation of aging? Proteomics (TMT+label-free) deciphers aging passwords
It is well known that stem cell aging is thought to be the root cause of tissue and organ aging, especially in biological systems with high turnover rates, such as hematopoiesis. As stem cells age, the potential for replacement of damaged tissue will also diminish. In humans, anemia, decreased adaptive immune system capacity, expansion of bone marrow cells at the expense of lymphocytes, and frequent blood systems Malignant tumors, which have been reported to be closely related to aging. But the mechanisms for these changes are still elusive. Scientists from the European Molecular Biology Laboratory, newly published in NATURE COMMUNICATIONS in 2018, are concerned about this direction. The primary purpose of this study is to use TMT and labelfree techniques to age the cell population in human hpc cells and bone marrow tick. Conduct research to reveal the molecular mechanisms of aging.
Source of literature
Https:// IF=12.353
research material
In 59 volunteers between the ages of 20 and 60, the cells obtained by puncture of the posterior tibial puncture were separated to obtain HPC cells, and five other cells associated with bone marrow tick: LYM (lymphocytes and precursors) , MON (monocytes/macrophages and precursors), GRA (granulocyte precursor), ERP (erythrocyte precursor), MSC (mesenchymal stem cells/wd stromal cells).
Technical method
TMT marker quantitative proteome and label free graded non-labeled quantitative proteomics (see figure below). Transcriptomics studies provide a blueprint for the underlying molecular mechanisms and indicate that genes associated with cell cycle, bone marrow lineage, and myeloid malignancies are up-regulated in aged HPC compared to younger HPCs. Based on the quantitative method of TMT, the changes in aging process in the same cell population were accurately determined, and the label abundance (LF) method was used to evaluate the protein abundance between different cell populations. This is also an innovation in this paper, using two quantitative omics to separately analyze changes in protein levels within and between cell populations. The single-cell sequencing of HPCs was also performed in conjunction with the Single-cell RNA-sequencing method to detect changes in the transcription levels associated with significantly altered proteins in the proteome.
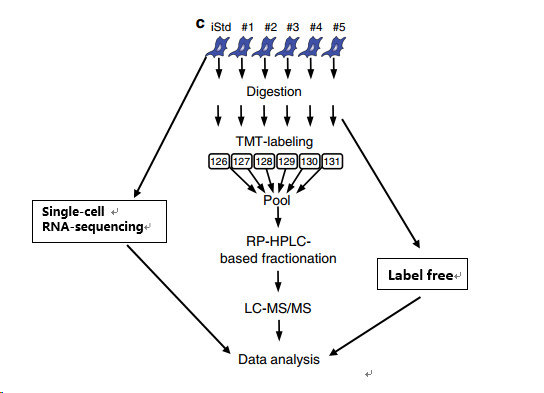
Result analysis
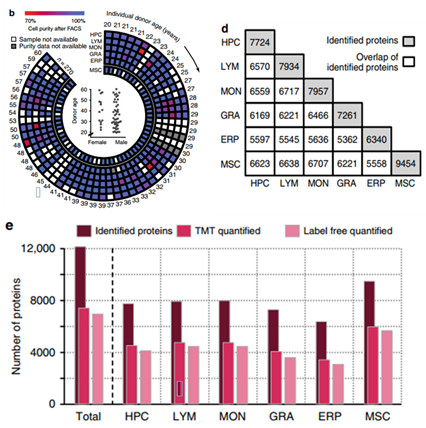
1. Identification of the results of the identification of the protein
The 6 kinds of cells isolated were subjected to label free and TMT quantitative analysis, and a total of 12158 proteins were identified, and the number of identified cells and the overlap between them were displayed.
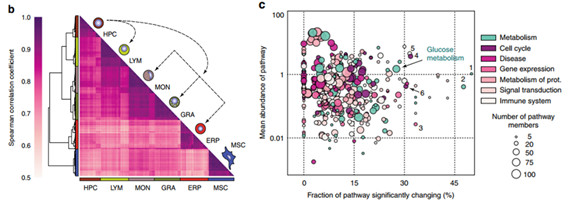
2. Cluster analysis of the data obtained from label free , combined with the Spearman correlation coefficient, the results are shown in Figure b below, it can be seen that the expression of commonly expressed proteins can be used to distinguish six different cell populations, which may reflect different The cell line is tailored to the specific metabolic requirements of the lineage and to the specific processes and functions of the cell. In order to understand whether the abundance of pathways in different cell populations is different, the authors analyzed the pathways of LF data based on the Reactome database. Figure C below shows the results of pathways. Through data integration analysis, some major proteins involved glycolytic pathways. .
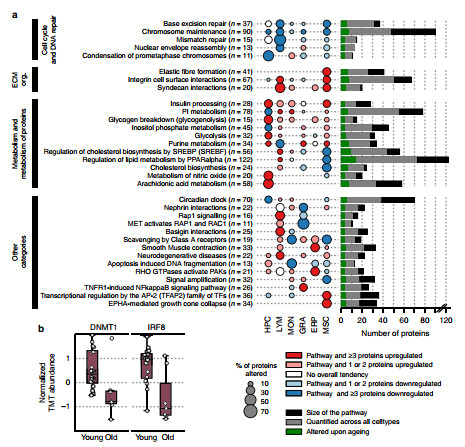
3. The effect of age on the proteome
To investigate the relationship between age-enhancing and proteomic changes, the authors performed Spearman's correlation analysis on TMT data and performed pathway analysis based on the Reactome database, which revealed significant cell type-specific and age-related correlations in protein abundance. The changes, as shown in panel a above, show 109 pathways screened based on the five highest up- and down-regulation pathways in each cell population. The authors also found changes in some of the reported aging markers. For example, in aged HPC cells, both IRF8 and DNMT1 are reduced.
4. Age affects carbon metabolism in HPC cells
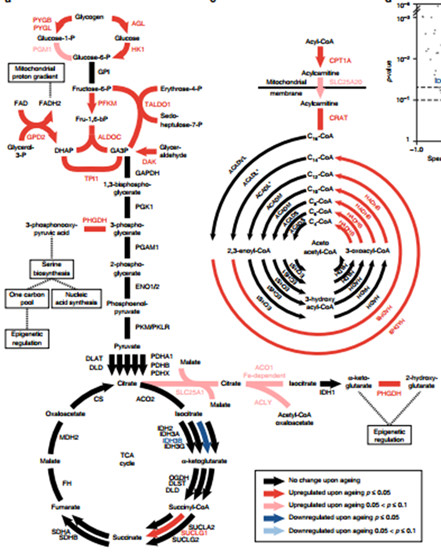
Through analysis, the authors found that the most noteworthy change in HPC cells caused by age is the change in enzymes, which play an important role in glycolysis, glycogen catabolism, and fatty acid beta oxidation. The central carbon cycle undergoes significant changes in aging. Figure a depicts glucose metabolism and TCA cycle in HCP cells. Arrows represent but one-way reactions. Strokes represent two-way reactions. Different colors represent different expressions. Black indicates that the enzyme does not Age changes, red and blue represent enzymes that are significantly up-regulated and down-regulated, respectively. Eventually, 17 proteins were found to have undergone significant changes in the glycolysis and TCA cycle pathways (consider the metabolomics data).
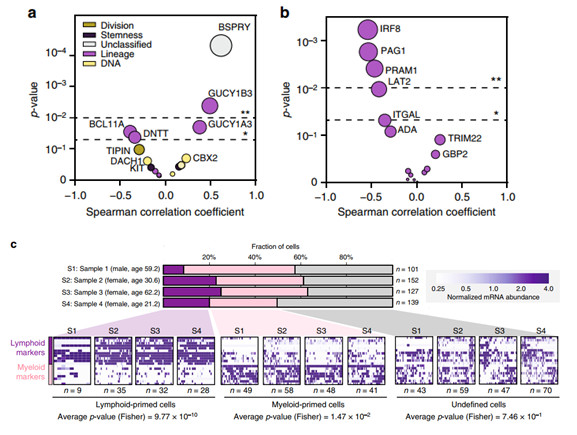
5. Comparison of myeloid and lymphoid differentiation during aging
In addition, the authors also found that bone marrow cells and lymphocytes differentiate more with age. The 17 proteins that are clearly found in the HPC population are involved in maintaining pluripotency or differentiation along the myeloid or lymphoid lineage. To describe the age-related functional attenuation of HPCs, the authors subsequently examined the dynamics of abundance of these proteins with age, and found that two proteins (DNTT and BCL11A) that are directly related to lymphoid development and function are significantly reduced with HPCs aging. Conversely, the downstream signaling effectors GUCY1A3 and GUCY1B3, with a significant increase in aging of HPCs, have been shown to regulate hematopoiesis in nitric oxide-cyclic guanylate pathway signaling, and may also indicate differentiation toward the myeloid lineage.
In order to examine whether the increase in enzyme abundance during the preparation phase of the glycolytic pathway may be a direct consequence of the differentiation of the CD34+ cell lineage into the bone marrow, the authors analyzed subjects from young (n = 2) and elderly (n = 2). Transcriptome results of 519 single-cell sorted HPCs. Each individual HPC cell is classified based on the abundance level of messenger RNA (mRNA) markers of lymphoid or myeloid differentiation (panel c). It was found that the mRNA levels of aged glycolytic enzymes in bone marrow-primed HPCs were higher than those induced by lymph, while the transcripts of enzymes not affected by age maintained similar levels in the two subsets.
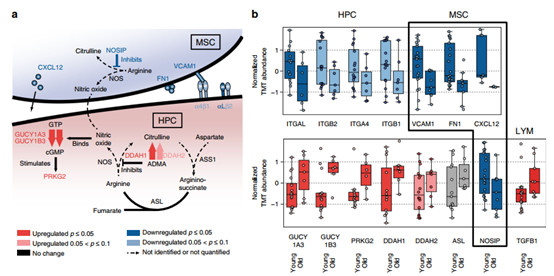
6. Changes in bone marrow tick associated with HPCs
Several homing and effervescent essential factors and adhesion molecules responsible for HPCs produced by the cellular microenvironment have decreased abundance during aging of MSCs, whereas stromal cell-derived factor-1 (SDF-1/CXCL12), vascular cells Adhesion molecule 1 (VCAM1) and fibronectin (FN1) were enhanced in aging when MSCs were aged. These changes indicate that aging is associated with recombination of ECM and structural changes in the structure of the bone marrow tick.
summary
This article is mainly through the classic TMT and label-free high-throughput omics, combined with single-cell sequencing to study the differentiation and functional decline of HPC cell population during aging and the bias of bone marrow differentiation, and in-depth analysis of different age tables Differences in type and function, revealing the changes in bone marrow tick caused by aging, and at the same time causing the decline of HPC homing pathways, providing a strong theoretical basis for exploring the mechanism of aging, and providing research in later studies. Ideas and directions.
Protein|modification|metabolism|lipid|structural confirmation
T: 021-54665263
E:
Q: 1875681852
Virus Specimen Collection Tube
Inspection principle:
It can perform protein denaturation on fresh clinical virus samples to inactivate the virus, prevent secondary transmission of infection, and ensure the safety of transportation and testing personnel.
♣.Structural composition: Combination of cotton swab and transport medium (VTM).
♣. Product requirements:
The product should be airtight, avoid high temperature, avoid direct sunlight storage. It should be used in a clean, hygienic, pollution-free, and temperature-friendly environment.
♣, Storage conditions and validity period:
â‘ , the product should be stored in a clean, dry and ventilated environment,
②, the temperature is 5℃-35℃;
â‘¢, relative humidity <85%RH;
â‘£, product shelf life: 12 months.
♣. How to use
â‘ Before sampling, mark relevant information on the label of the sampling tube.
â‘¡. Sampling with the corresponding cotton swabs.
â‘¢ After the collection is completed, quickly put the cotton swab into the collection tube, break the part higher than the sampling tube, and tighten the tube cover.
â‘£. For the specific sampling method, please refer to the following:
a) Nasal swab Gently insert the sampling head into the nasal cavity, stop for a while and then slowly rotate to exit, immerse the collected specimen in the Xiangxiang solution, break the excess part and discard it, and tighten the sampling tube cover.
b) Pharyngeal swab: Wipe bilateral pharyngeal tonsils and posterior pharyngeal wall with the sampling head, immerse the collected specimen in the sampling solution, break off the excess part and discard it, and tighten the cap of the sampling tube.
c), Mycoplasma Chlamydia, Ureaplasma specimen collection
Male: Insert the sampling head into the urethra about 2cm and rotate, stay for a while and then exit, and immerse the collected specimen in the sampling solution.
Female: Wipe the mucus of the cervical orifice, insert the sampling tip into the cervical canal for 1-2 cm for sampling, immerse the collected specimen in the sampling solution, break off the excess part and discard it, and tighten the cap of the sampling tube.
♣. Precautions
1. After the virus is collected, the disposable sampling swab should be completely inserted into the preservation solution, so that the virus can be retained to the greatest extent possible.
â‘¡ The collected specimens must be sent for inspection in time.
â‘¢. It is forbidden to use products with damaged packaging and expired validity period to prevent pollution.
This single-use Virus Sampling Tube is used for in vitro diagnosis. It cannot be used for human or animal oral or external use. If swallowed, it may cause serious events; it is irritating to eyes and skin. If it is not splashed into the eyes, rinse with water.
Virus Sampling Tube,Virus Specimen Collection Tube,Viral Transport Tube,Saliva Virus Sampling Kit
Jilin Sinoscience Technology Co. LTD , https://www.jilinsinoscience.com