Identification of multi-site molecular ions in pig muscle by T-wave ion mobility mass spectrometry
Identification of multi-site molecular ions and their fragment ions of fluoroquinolone antibiotics in pig muscle by T-wave ion mobility mass spectrometry
Mike McCullagh1, Sara Stead 1 , Jonathan Williams 1 , Wouter de Keizer 2 and Aldert Bergwerff 2
1 Waters Corporation (Manchester, UK) 2 RnAssays BV (Utrecht, The Netherlands)
Advantages Application of separation using orthogonal <br> T-wave generated by the ion mobility help analyze complex matrices.
â– Orthogonal mobility separation technology can acquire and generate MS precursor and MSE ion spectra of individual components in complex samples.
â– For the observed accelerator, it can be reconfirmed and used as an additional identification point.
â– Using HDMSE as an analytical tool, you can gain a deeper understanding of the differences between research in the laboratory or in the laboratory.
Waters Solutions
ACQUITY UPLC® System
SYNAPT® G2-S mass spectrometer
MassLynx® software
DriftScopeTM ACQUITY UPLC BEH Column
Introduction
Fluoroquinolone is a class of antimicrobial agents that can be applied to livestock for a variety of purposes, including: (a) preventing and controlling infection; and (b) promoting growth. Concerns about the spread of antagonistic microorganisms in humans, the US Food and Drug Administration (FDA) in September 2005 issued a ban on the use of 1,2 enrofloxacin and ciprofloxacin in livestock production. Since 2006, the European Union (EU) ban on the use of antibiotics in livestock growth promoters (AGP), the same year, the last four kinds of antibiotics as growth promoters is prohibited 3.
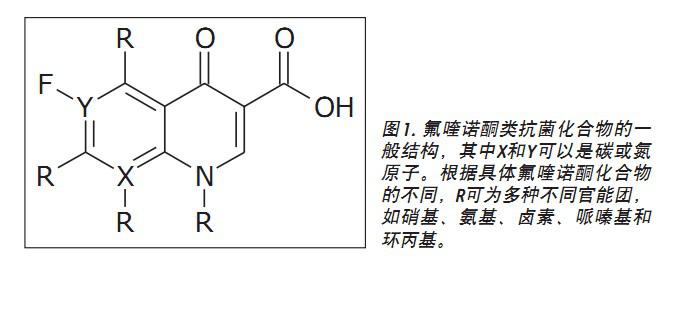
Although fluoroquinolones are a class of chemically diverse amphoteric compounds, these compounds all have a 4-quinolone ring basic structure. In order to enhance its antibacterial and pharmacokinetic properties, various modification attempts have been made, including the introduction of different functional groups around the quinolone ring (benzopyrone nucleus). The fluoroquinolone has a fluorine atom at the 6 position of its bicyclic structure (Fig. 1) and exhibits broad-spectrum antibacterial activity. At present, eight predetermined (fluoro) - Maximum Residue EU quinolone compound (the MRL), depending on the species and tissue types, 4 limited range between 10 and 1900 μg kg-1.
experiment
Preparation for extraction
The porcine muscle tissue extract used in this study was generously provided by RnAssays. Simply put, before the extraction, according to the relevant EU maximum residue concentration requirements, add 25 different antibacterial compounds (from fluoroquinolone, tetracycline and amide alcohol) to the known blank pig muscle, and put it in water/organic In the extraction solvent, it is mechanically ground into a homogenate and then centrifuged. The supernatant was taken and placed in an autosampler vial for subsequent LC/MS analysis.
UPLC conditions <br> system: ACQUITY UPLC
Column: ACQUITY UPLC BEH C18 1.7 μm, 50 × 2.1 mm
Column temperature: 40 °C
Flow rate: 0.6 mL/min
Mobile phase A: water (0.1% formic acid)
Mobile phase B: acetonitrile (0.1% formic acid)
Injection volume: 10 μL
<br> MS conditions MS: SYNAPT G2-S
Ionization mode: 2.0 kV ESI+
Taper voltage: 25 V
Desolvation gas temperature: 550 °C
Reference mass: leucine enkephalin, [M+H] + =556.2771
Collection range: 50-1200 m/z
Acquisition rate: 4 spectra / sec collision energy: 15-45 eV
Resolution: 20,000 FWHM
IMS T-Wave speed: 550 m/s
IMS T-Wave pulse height: 40 V
Buffer gas: N 2 and CO 2
Typical steps for fluoroquinolone residue analysis are: first solvent extraction followed by solid phase extraction (SPE) purification and separation by LC, combined with UV detection, fluorescence (FL) or mass spectrometry (MS). These methods typically only detect a small amount of target analyte and the sample throughput is low 5 . Many different types of mass analyzers are often used for Veterinary Drug Residue (VDR) analysis, including single quadrupoles, tandem quadrupoles, ion traps, and more recently time-of-flight (Tof) based technologies 6,7,8 . The use of tandem quadrupole mass spectrometers instead of single-stage mass spectrometry for quantitative analysis is now widely accepted because it offers superior performance advantages in terms of selectivity and sensitivity. This is due to the multiple reaction monitoring (MRM) mode, in which the first quadrupole is used first to select the mass of the parent ion. When the mass of the specific parent ion is selected, it breaks into the collision cell and finally Detected with a second quadrupole. Even with this approach, a small fraction of other analyte-independent compounds can produce interfering signals. For this reason, a second pair of MRM ion pairs is monitored. Only in the sample, the MRM of both pairs of ion pairs produced a chromatographic peak and the retention time of the chromatographic peak was consistent with that of the standard compound to determine the presence of the compound in the sample. In addition, the intensity ratio of the two MRM peaks must also be the same as for the pure standard. This method has been further refined in European Directive (2002/657 / EEC), the instruction specifies requirements for the quantitative determination and analysis of food and animal feed 9 of veterinary drug residues.
Tandem quadrupole mass spectrometers are widely used in residual monitoring projects that require highly sensitive detection (usually at lower concentrations of μg kg-1) in complex matrices. When selecting a new tandem quadrupole-based method for veterinary drug residue analysis, the selection of the MRM channel is critical and must be selected and validated in accordance with the 2002/657/EC guidelines described above.
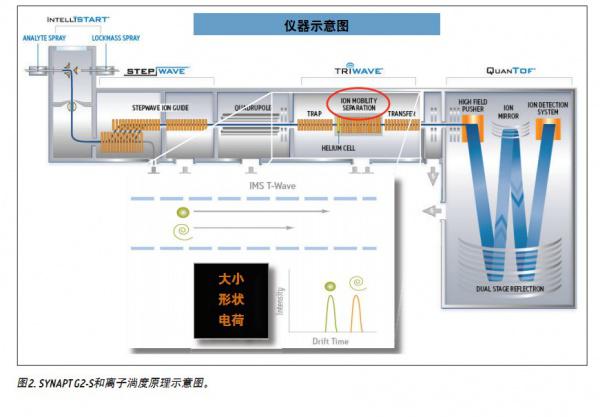
This application summary explores the use of High Definition Mass Spectrometry TM (HDMS TM ) as an important way to develop tools to support the unequivocal identification of tissue crude extracts of fluoroquinolone antibiotics. Crude extracts of porcine muscle tissue were analyzed using HDMS to determine the presence of antibiotic residues including fluoroquinolones. This technology has unique advantages for analyzing complex matrices. It combines high-resolution mass spectrometry with an efficient separation method based on ion mobility. Ion Mobility Spectroscopy (IMS) is a fast orthogonal gas phase separation technique that provides separation in another dimension within the allowable range of LC separation time. It distinguishes compounds based on their size, shape, and charge. In addition, in the HDMS experiment, the parent ion and its fragment ion information can be obtained in a single injection, called HDMS E.
Results and discussion
Using the general gradient conditions, the antibiotic ciprofloxacin was eluted at a retention time of 2.19 minutes, as shown in Figure 3 (Figure 3 is the base peak ion chromatogram).
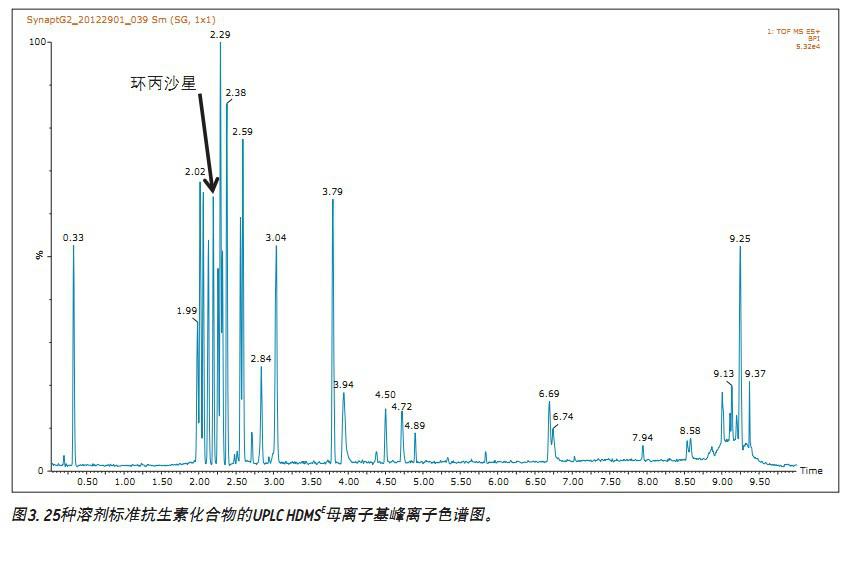
Figure 4 shows a conventional accurate mass spectrum obtained using MassLynx software, where it was observed that the accurate mass measurement error of [M+H]+ ions was 0 ppm at m/z of 332.1410. By obtaining accurate mass measurements and using the elemental composition calculation tool in MassLynx, the elemental composition is obtained, enabling us to reliably identify 2.19 minutes of peaks.
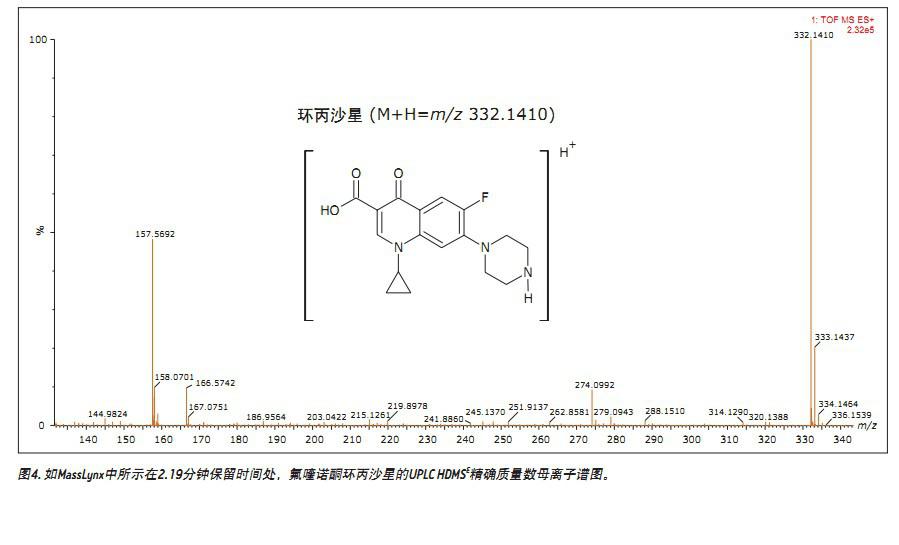
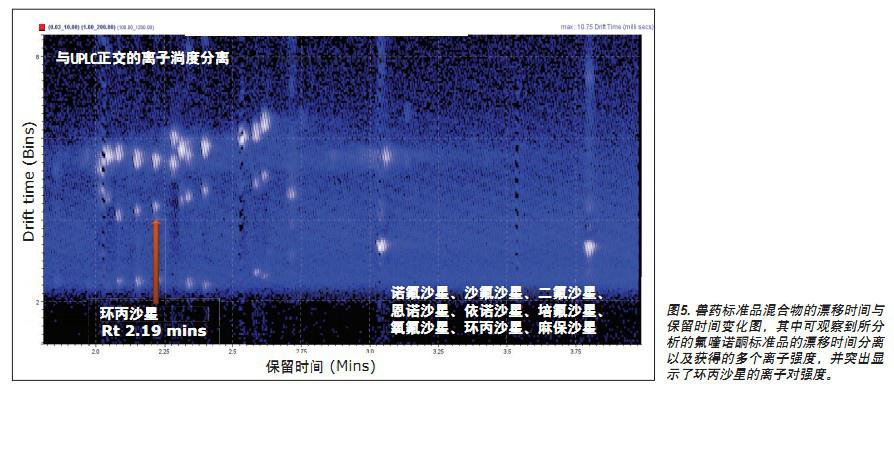
The conventional data of Figures 3 and 4 illustrate that the fluoroquinolone analyzed is a single component. However, using ion mobility analysis, it was found that the fluoroquinolone is composed of two ionized materials, as shown in FIGS. 5 and 6. Although the two components differ only in the protonation sites, the separation was 1.14 milliseconds in the analysis of ciprofloxacin using ion mobility. The temperature separation can be viewed using the DriftScope in Figure 5, which is a plot of drift time and retention time for a mixture of veterinary drug standards. Figure 5 shows the drift time separation of all fluoroquinolone standards analyzed and the ionic strength obtained. The ion pair strength of ciprofloxacin is also highlighted. Figure 6 shows more specific data for this pair of ions, highlighting the corresponding acid/base group protonation sites and drift time 10 .
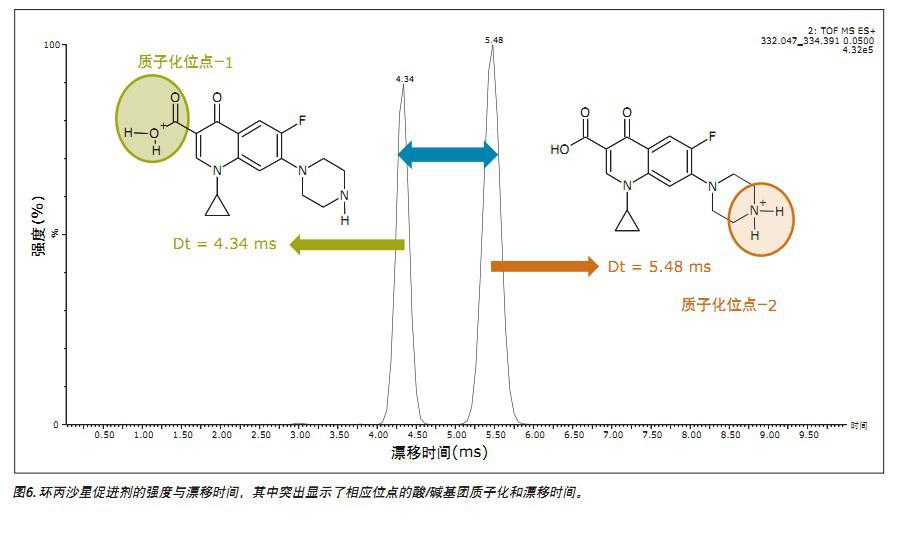
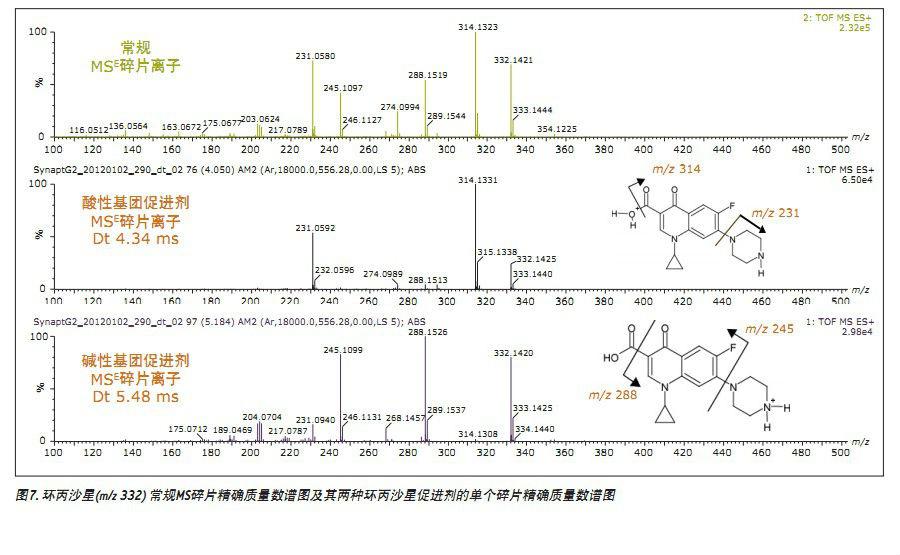
Therefore, each accelerator is treated as a single component to obtain a respective fragment spectrum (Fig. 7). From the single component MSE fragmentation spectrum, two kinds of temperature-separated substances produced by the protonation of the acidic and basic groups of ciprofloxacin can be determined. In fact, the fragment ions of m/z 314 and m/z 231 (Fig. 7) are produced by ciprofloxacin ionized by acidic groups. The m/z 288 and m/z 245 fragment ions are only produced by ciprofloxacin protonated with a basic group. It has been observed that both promoters form m/z 231 fragment ions. Further research on fragment ions has been completed and will be presented in another article.
In Figure 4, a conventional MS spectrum generated by the UPLC HDMS E experiment is shown. At m/z 157 and m/z 166, it is clear that there are two small double-charged ions in ciprofloxacin, indicating that ciprofloxacin does form a double-charged species. Separation can be achieved using ion mobility and confirm that the small molecule forms a double charged species. The formation of a double-charged substance depends on the MS parameters used, especially the cone voltage. If the cone voltage is too high, no double-charged species can be observed and only [M+H] + can be observed.
The data obtained indicate that method development and selected analytical methods should be carefully considered because the ratio and formation of the promoter will vary depending on the eluent flow rate, capillary voltage, cone voltage, and matrix. If the MRM method is selected, the experimental conditions used and the specific mass spectrometry channel selected should be considered. The experimental data show that if the selected MRM ion pairs are inconsistent, it is easy to cause deviations in the laboratory and between different laboratories, and the challenges encountered in maintaining the reproducibility results of these compounds are explained. Ion mobility is a powerful tool for method development that ensures method reliability and consistent results.
In addition to more specific and reliable method development, different drift times of different components can be used as another identification condition. This application note describes retention times, accurate masses of parent ions, accurate masses of fragment ions, and two drift times that can be used as ciprofloxacin identification conditions.
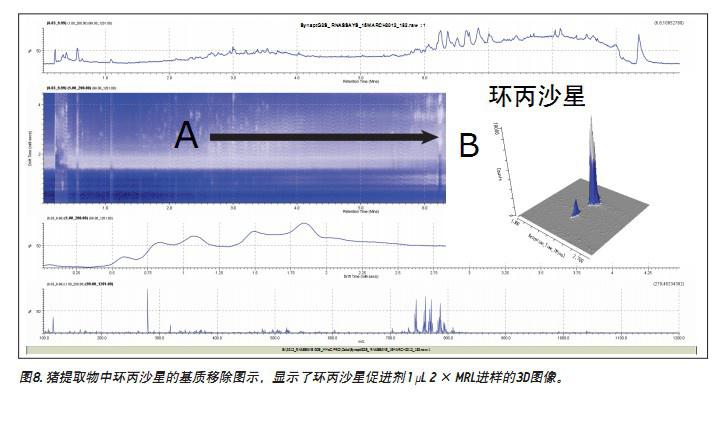
In addition to using ion mobility to obtain a new identification condition, orthogonal separation resulting from ion mobility can also be used for spectral cleanup. The drift time and retention time changes (Fig. 8A) show the ionic strength, expressed in white pixels. The range of presence of matrix ions is indicated by a continuous white color. The more intense analyte and matrix components are represented by more pronounced white spots. However, due to the large amount of ionic strength produced by the matrix, it is difficult to observe low concentrations of target analytes. In Figure 8B, a ciprofloxacin accelerator is extracted from the porcine matrix. A single component of MS and its fragment ion spectra can be produced based on clear, selective separation using ion mobility. Under these conditions, the ratio of the acidic site promoter to the basic site promoter in the porcine matrix was determined to be 5:1. This is affected by capillary voltage, cone voltage, electrode position, flow rate, and matrix. In the injection experiment, the ratio of the acid/base accelerator can be changed, and even the highest level accelerator can be changed.
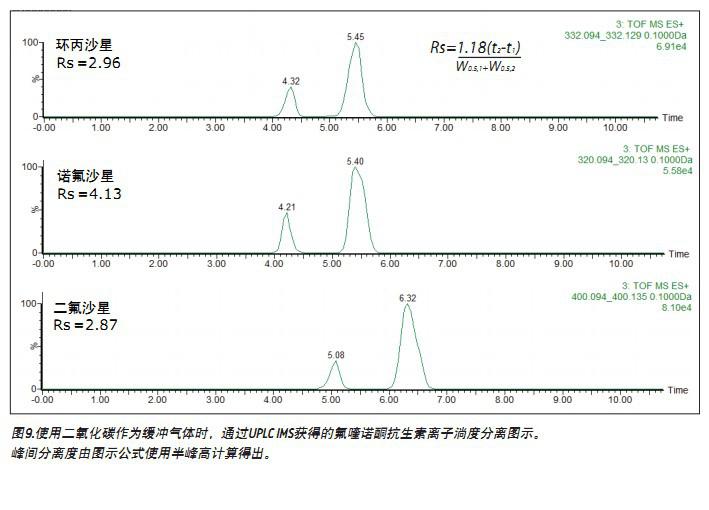
Figures 9 and 10 show the distribution of the time of arrival of ciprofloxacin, norfloxacin and difloxacin accelerators, and the inter-peak resolution calculations obtained using nitrogen and carbon dioxide as buffer gases, respectively. Although both gases achieve acceptable resolution, an Rs value between the peaks greater than 1.5 is considered complete separation. Previous studies have suggested that the use of these two buffer gases alternately increases the resolution of ion mobility by 10-14 .
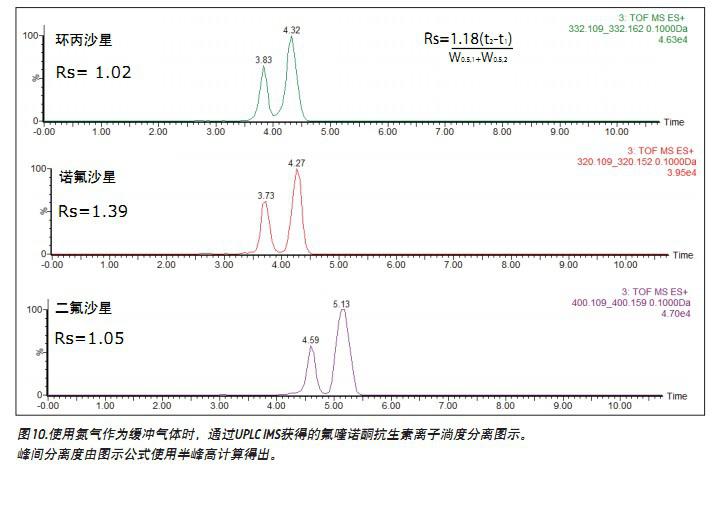
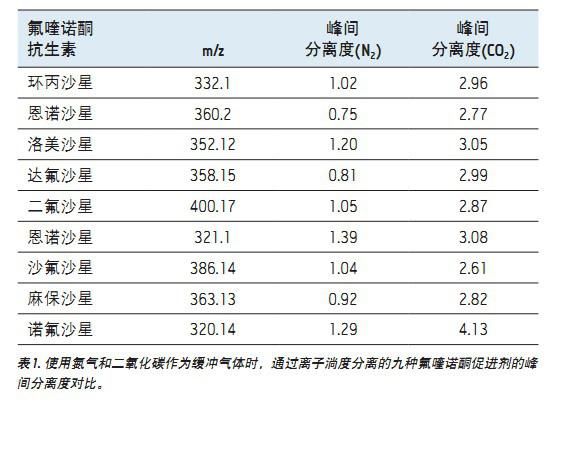
When performing IMS, ion separation is performed in a T Wave ion mobility (TWIM) drift tube. The separation results are determined by the charge state, mass number, shape, buffer gas polarizability, and the interaction between ions and neutral gas molecules. In this application, increasing the polarizability of the buffer gas increases the separation capacity (peak capacity) of the TWIM. Therefore, the degree of separation between peaks and peaks can be improved by fully considering the polarizability of the buffer gas used. In the present study, carbon dioxide was used. The peak-to-peak resolution (Rs=1.18(ta-tb)/W0.5, a+W0.5,b) obtained using nitrogen or carbon dioxide is summarized in Table 1 (where Rs represents the calculated resolution, W0. 5a and W0.5b represent the half-peak widths of peaks A and B, respectively, and ta and tb are the drift times of peaks A and B, respectively). It can be seen that when carbon dioxide is used as the ion buffer buffer gas, all the fluoroquinolone promoter pairs are completely separated, and the peak-to-peak resolution is between 2.61 and 4.13. The increased peak capacity of carbon dioxide further enhances the quality of the single component parent ion and its corresponding single component fragment ion spectrum.
in conclusion
Based on the observation of the characteristic ionization of fluoroquinolone compounds in this study, it is reasonable to use UPLC IMS MS E for method development.
â– Using ion mobility, the magic separation of the same molecule and different protonated substances can be achieved.
â– Display and identify fragment spectra of the same molecule and different sites of proton compounds.
â– Obtain the parent ion MS and its MS E fragmentation spectrum for each component â– The HDMS E observations can explain why the results of the study reported by the specific MRM ion team in the same laboratory are sometimes different.
â– The drift time value can be used as another qualification condition in addition to the retention time, the precursor mass accurate mass, and the fragment accurate mass spectrum.
â– Ion mobility separation effectively separates target peaks from matrix interference without the need for purification and chromatographic separation of complex samples.
references
1. Final Decision of the Commissioner, Docket # 2000N-I 57 1 October 24, 2000, Withdrawal of the approval of the new animal drug application for enrofloxacin in poultry. Department of Health and Human Services, US Food and Drug Administration.
2. FDA Center for Veterinary Medicine, June 2, 1997, FDA Order Prohibits Extralabel Use Of Fluoroquinolones And Glycopeptides.
3. Official Journal of the European Union, L268: 29-43, Regulation (EC) No 1831/2003 of the European Parliament and of the Council of 22 September 2003 on the use of animal nutrition.
4. The Official Journal of the European Union, L24:1-8 Annex I, Council Regulation (EEC) No 2377/90 of 26 June 1990 laying down a Community procedure for the establishment of maximum residue limits of veterinary medicinal products in foodstuffs of animal Origin.
5. Verdon E, Couedor P, Roudaut B, Sandérs PJ. Multiresidue Method for Simultaneous Determination of Ten Quinolone Antibacterial Residues in Multimatrix/Multispecies Animal Tissues by Liquid Chromatography with Fluorescence Detection: Single Laboratory Validation Study. AOAC Inter. 2005; 88:1179 -1192.
6. Kaufmann A, Butcher P, Maden K, Widmer M, Giles K, Uria D. Are liquid chromatography/electrospray tandem quadrupole fragmentation ratios unequivocal confirmation criteria? Rapid Commun. Mass Spectrom. 2009; 23: 985-998.
7. Mol HG, Zomer P, de Koning M. Qualitative aspects and validation of a screening method for pesticides in vegetables and fruits based on liquid chromatography coupled to full scan high resolution (Orbitrap) mass spectrometry. Anal Bioanal Chem. 2012; 403: 2891-2908.
8. Croley TR, White KD, Callahan JH, Musser SM. T he chromatographic role in high resolution mass spectrometry for non-targeted analysis. J Am Soc Mass Spectrom. 2012; 23:1569.
9. Commission Decision (2002/657EEC) Official Journal of the European Communities 2002. 10. Lalli PM, Iglesias BA, Toma HE, de Sá GF, Daroda RJ, Silva Filho JC, Szulejko JE, Araki K, Eberlin MN. Protomers: Formation, separation and characterization via travelling wave ion mobility mass spectrometry. J Mass Spectrom. 2012; 47(6):712-9. 11. Eberlin MN, Lali PM, Nachtigall FM, Riccio MF, de Sa GF, Daroda RJ, de Souza V, Campuzano I, Souza GHMF. SYNAPT HDMS: Improving Ion Mobility Separation by Increasing Drift-Gas Polarizability. Waters Technical Note 720003201en. 2009 October. 12. Jurneczko E, Kalapothakis J, Campuzano IDG, Morris M, Barran PE. Effects of Drift gas on collision cross sections of a protein standard in linear drift tube and traveling wave ion mobility mass spectrometry. Anal Chem. 2012; 84(20):8524-31. 13.Asbury GR, Hill HH. Using different drift gases to change Separation factors (alpha) in ion mobility spectrometry. Anal. Chem. 2000; 72(3): 580-4. 14. Fasciotti M, Sanvido GB, Santos VG, Lalli PM, McCullagh M, de Sá GF, Daroda RJ, Petera MG, Eberlina MN. J Mass Spectrom. 2012; 47(12):1643-7.
Disposable Drape Sheets,Disposable Medical Sheet,Disposable Medical Bed Sheets,Disposable Hospital Bed Sheets
Henan Anbang Medical Supplies Co., Ltd. , https://www.anbangmedical.com