Application of two-color synchronous imaging in imaging experiments such as fluorescence co-localization
2023-03-15 10:10:41
Fluorescence co-localization is a very common technique in today's biomicroscopy. Two or more fluorescent probes of different colors are used to label different structures / sites so that their relationships are clearly combined. Show on the image. As researchers become more demanding on experiments, these fluorescence co-localization imaging is expected to be used in experiments where the fluorescence intensity changes at high speed or the position of the sample itself is changing, such as active zebrafish and nematodes. Fluorescent co-localization imaging of cells. In these experiments, since the sample itself is moving, the shorter the imaging time interval between the two colors, the more it can reflect the true condition of the co-localization of the fluorescent probe.
In other experiments, the fluorescence color ( wavelength ) of the same fluorescent probe changes with the environment, then the ratio of the fluorescence intensity of the two colors can reflect the environmental changes, such as Di4 to the cell membrane potential. , Cameleon to Ca ion concentration and so on. When the relevant environment changes faster, the shorter the imaging time interval between the two colors, the more accurate the measurement.
Combining the above two types of imaging experiments, it is necessary to shoot two different color fluorescent signals at the same time as possible. The ideal situation is the two-color synchronous imaging mentioned in this Application Notes , that is, completely simultaneous Photograph the fluorescent signals of the two color channels.
Two-color simultaneous imaging - fully synchronized imaging with W-View GEMINI
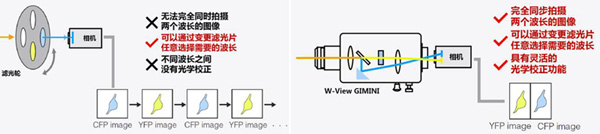
The most direct idea of ​​two-color synchronous imaging is to use a color camera, but most color cameras today add a filter coating (usually red, green, and blue) to each pixel based on the black and white chip, as shown below. ), the color filter coating allows different pixels to obtain different color information, and finally a color picture is synthesized. Such a design is indispensable in brightfield imaging (eg, HE staining, immunohistochemical sectioning), but imaging of bioluminescent signals is deficient. First, the appearance of the filter coating absorbs or reflects part of the incident light signal, reducing the sensitivity of the entire chip, affecting the imaging signal-to-noise ratio in the weaker fluorescence imaging of the signal; secondly, due to the different colors before the different pixels The filter coating, the actual resolution for monochromatic optical signals, such as fluorescent signals, will be reduced. In other words, for a 1.4 megapixel color CCD camera, the pixels that can actually detect the green fluorescent signal are only about 700,000 pixels, and the remaining 700,000 pixels of green light intensity information are calculated. Because green is the most sensitive color of the human eye, in general, a color camera is composed of 4 pixels, two pixels are green, and the other two pixels are blue and red. More importantly, if you use this type of color camera for two-color fluorescence synchronous imaging, some fluorescent colors that are not red, green, and blue (corresponding to the three filter coatings on the color camera pixels) will be greatly affected. limit.
So traditionally, many laboratories switch between different colors by switching the filter block on the microscope or using a filter wheel. However, it is impossible to completely capture the two colors of the picture at the same time. The switching time of the filter block turntable takes about 1 second, and each switch between the different filters of the filter wheel takes tens of milliseconds. It affects the time resolution of the experiment, so in the case of high-speed proportional imaging, etc., the filter wheel or the filter block turntable is easy to cause data error (for example, please refer to "Application Example 1 " shown below ).
With the Hamamatsu W-View GEMINI, there is no such concern. The two colors of the signal are imaged onto the upper and lower halves of the camera, enabling full synchronization of the two color images.
Two-color synchronous imaging - one Flash 4.0 LT camera for two
Using a two-color spectroscopic accessory such as the W-View GEMINI to image two colors of signals onto a single sensor of a camera, the time for simultaneous imaging is well solved, but for most cameras, the entire sensor can only be used. Setting an exposure time, when the signal strengths of the two colors differ greatly, it will be difficult to simultaneously ensure the imaging signal-to-noise ratio of the two colors at an optimal state.
The Hamamatsu Flash 4.0 LT can adjust the exposure time of the upper and lower halves of the same chip. So when using W-View GEMINI with Flash 4.0 LT , we can very flexibly adjust the relative brightness of the two color signals to get a picture that better highlights the desired signal and structure. When the signal difference between the two color channels is very large, the flexible exposure time setting of Flash 4.0 LT + W-View GEMINI can set different exposure times for different wavelengths while ensuring the signal-to-noise ratio of the two wavelength signals. .

W-View GEMINI Application Example 1 - High-speed calcium ion imaging of HeLa cells expressing Cameleon (YC3.60) upon histamine stimulation
Objective lens: 100x , NA 1.49 | Frame rate: 33.3 frames / sec |
Camera: Hamamatsu ImageEM X2 EMCCD | Exposure time: 30 ms |
Accessories: Hamamatsu W-View GEMINI | Gain: 1200x |
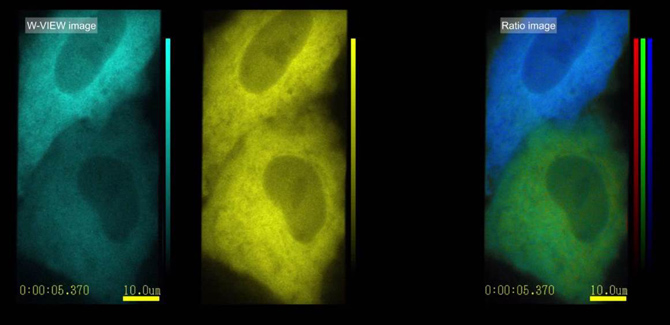
In the case of high-speed changes in intracellular calcium concentration, two-color simultaneous imaging can ensure high-speed data acquisition. Taking this experiment as an example, the molecules of YC3.60 contain two parts, CFP and YFP . When there is no calcium ion binding, the two parts are far apart. When excited by 430nm light, the 480nm cyan signal emitted by CFP is obtained. ; when combined with calcium ion, YC3.60 two portions of the CFP and YFP will wait until the molecule together, meet the conditions of FRET occurring CFP-YPF, 430nm when excitation light is irradiated, the energy transferred by the CFP to YFP, emission the yellow fluorescence signal of 535nm. Therefore , the intracellular calcium concentration can be monitored by detecting the signal intensity ratios of 480 nm and 535 nm in real time .
If the filter wheel is used, it is assumed that the switching time of different filter positions is 30ms (this is already a good product), CFP and YFP each require 30ms exposure time, one data point needs to switch the filter position twice (such as Switching from CFP to YFP and then switching back to prepare for the next data point), so it takes a total of 120ms to acquire a data point. The result is that we only get 8- bits per second when we use the configuration of the filter wheel. 9 data points (calcium ion concentration data).
With the W-View GEMINI , the fluorescence signals of CFP and YFP can be acquired simultaneously within 30ms of exposure time , and there is no time loss of color switching, which ultimately guarantees high-speed imaging of 33 frames per second, that is, per second. We can get 33 calcium ion concentration data points.
Both methods are highly deterministic in terms of kinetic performance.
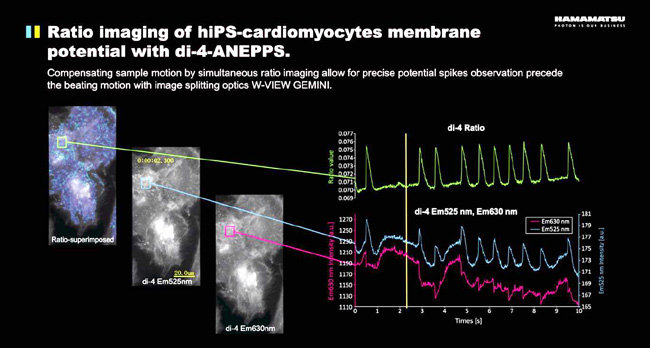
W-View GEMINI Application Example 2 - Di-4-ANEPPS using membrane potential change on cardiomyocytes imaging hiPS-
Objective lens: 100x , NA 1.49 | Frame rate: 100 frames / sec |
Camera: Hamamatsu Flash 4.0 V2 sCMOS | Exposure time: 10 ms |
Accessories: Hamamatsu W-View GEMINI | Emission wavelength: 525/50 nm & 630/92 nm |
In the case of a positional shift of the sample, two-color simultaneous imaging can offset the error caused by the change in sample position.
For example, in this case of inducing cardiomyocytes, we need to image the membrane action potential with the dye Di-4-ANEPPS . The fluorescent dye of Di-4-ANEPPS is excited by blue light ( ~488nm ), and its emission wavelength changes according to the membrane potential. By calculating the ratio of 525nm/630nm in different regions of the cell, the membrane potential of this region can be obtained. Variety. This imaging method can obtain a change in membrane potential at any position of the cell with respect to electrophysiological methods such as patch clamps, and the spatial resolution is higher. The W-View GEMINI two-color spectroscopic accessory and the high-speed, high-sensitivity Flash 4.0 sCMOS camera also ensure extremely high time resolution ( 100 data points per second ), allowing us to time the action potential at any position in the cell. The space is very accurate measurement.
                Â
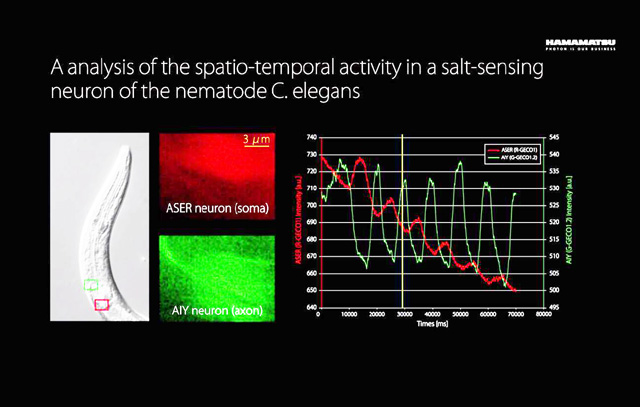
W-View GEMINI Application Example 3 - Analysis of the Temporal and Spatial Relationship of the Response of Salt-sensing Neurons in the Change of NaCl Concentration
Objective lens: 60x , oil | Frame rate: 30 frames / sec |
Camera: Hamamatsu Flash 4.0 LT | Exposure time: 33 ms |
Accessories: Hamamatsu W-View GEMINI | Probe: ASER-R-GECO1_AIY-G-GECO1.2 |
In this example, in order to monitor the performance of two neurons of ASER and AIY in the change of NaCl concentration outside the outside world , the researchers expressed the red and green calcium-sensitive fluorescent proteins R-GECO1 and G- on two neurons. GECO1.2 , then the NaCl concentration in the environment was switched between 0 mM and 50 mM in a 10 second cycle . By analyzing the changes in the concentration of calcium ions in the two neurons, it can be seen that the two nerve cells are opposite in different concentrations of NaCl .
Further reading: GCaMP calcium imaging, retinal neurons exhibit two opposite change in concentration of calcium ions (A high concentration when the B concentration is low, when the A B concentration decreased concentration becomes higher), how to use the method in a plan view Reslice Reflecting this relationship, please refer to the links in the article 14-16 : Click to enter to understand >> Â
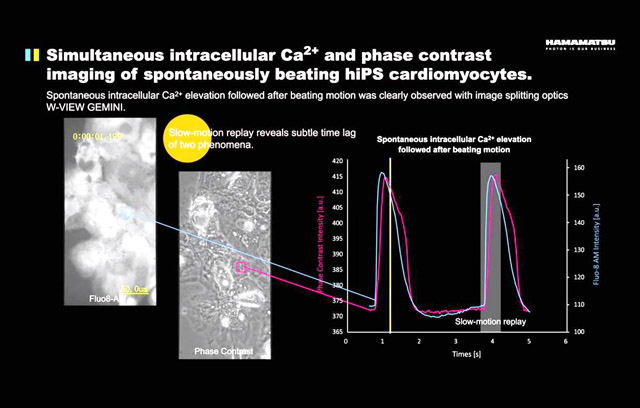
W-View GEMINI Application Example 4 - Analysis of changes in calcium ion concentration and cell shrinkage in time of myocardial cell pulsation
Camera: Hamamatsu Flash 4.0 V2 | Objective lens: 40x , NA 0.6 |
Accessories: Hamamatsu W-View GEMINI | Exposure time: 30 ms |
In addition to two different colors of fluorescence sync imaging, the W-View GEMINI can also be used for simultaneous imaging of fluorescence and brightfield. In this example, the researchers simultaneously monitored changes in calcium ion concentration and cell shrinkage during cardiomyocyte fluctuations. Luo- excited green-fluorescent Fluo-8 is used for calcium ion concentration monitoring, while bright-field phase contrast imaging uses 600-680 nm red light. By analyzing the calcium ion concentration and phase difference images in a single cell, it can be seen that the increase in calcium ion concentration is slightly ahead of the contraction of cardiomyocytes.
In this type of experiment, the signal transmitted by the bright field is much stronger than the fluorescent signal. In addition to adding a dimming film to the bright field transmitted light path to adjust the two signals, the more convenient method is to use Flash. 4.0 LT can be more flexible adjustment for the characteristics of the two half of the chip to adjust the exposure time (refer to the relevant paragraph of this article: two-color synchronous imaging - one Flash 4.0 LT camera for two).
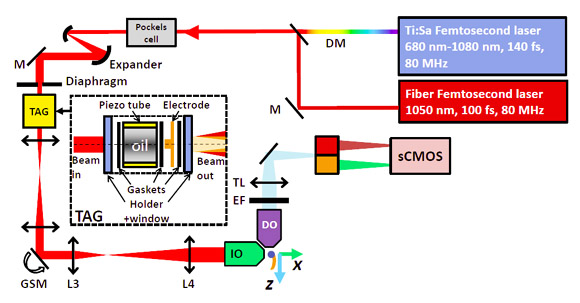
W-View GEMINI Application Example 5 - Zebrafish two-color two-photon lightsheet imaging
Camera: Hamamatsu Flash 4.0 V2 | Accessories: Hamamatsu W-View GEMINI |
At home, researchers at Peking University also used W-View GEMINI in their own two-color two-photon lightsheet imaging system. The system uses two femtosecond laser as a light source are two-photon excited fluorescence probe two colors, two-color three-dimensional imaging lightsheet. The sample is a 3- day-long zebrafish. Red and green fluorescent probes are labeled with red blood cells and islet cells, respectively. In order to minimize the acquisition time of the 3 -dimensional image, the movement of red blood cells in the blood vessels may affect the imaging quality. Two-color splitting with the W-View GEMINI allows the Flash 4.0 V2 camera to simultaneously image red blood cells and islet cells.
http://v.youku.com/v_show/id_XMTI3NjQzMjA1Mg==.html
Further reading : More information can be found in the submission of the Hamamatsu Micro Imaging Competition. The introduction of its unique high-speed 3D lightsheet imaging method 2P3A-DSLM can be found in its published paper .
More literature and application examples of two-color synchronous imaging
1. Single-molecule multi-color FRET, using Hamamatsu Flash 4.0 & W-View GEMINI, analysis of the interaction between ribosomes and tRNA by a combination of cy3/cy5/cy7:
Juette MF, et. al, The bright future of single-molecule fluorescence imaging, Curr Opin Chem Biol. 2014 Jun 20;20C:103-111.
Recommended Products




ORCA-Flash4.0 V2 | ORCA-Flash4.0 LT | IMagEM X2 | W-VIEW GEMIN I |
-10°/-30° deep cooling and >70% QE high speed and high sensitivity sCMOS | > 70% QE of high speed and high sensitivity sCMOS <br> | The industry's highest frame rate ( 70fps @ 512x512 ) back-illuminated EMCCD | Two-color spectroscopic accessory |
Yancheng Rongtai Labware Co.,Ltd , https://www.shtestlab.com